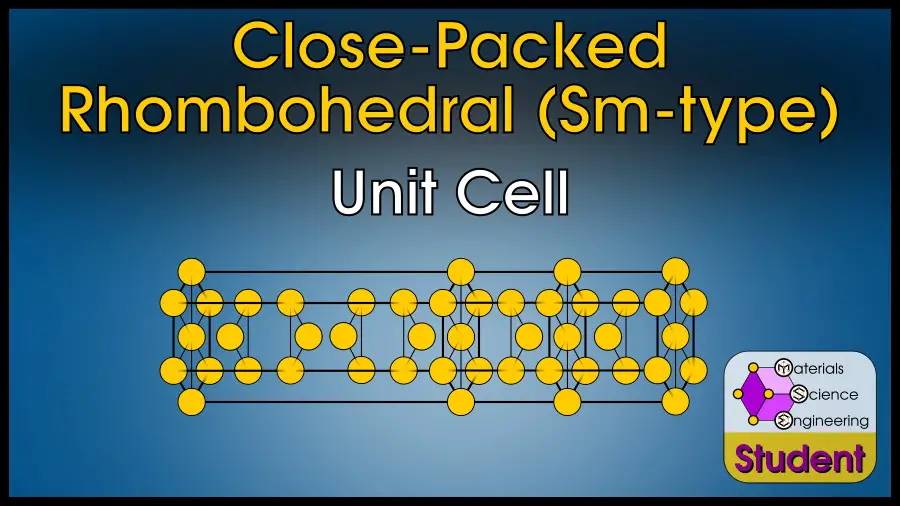
The Close-Packed Rhombohedral (abbreviated CPR in this article) is more commonly known as the Samarium-type crystal, because Sm is the first element to display this crystal structure. Technically, there are infinite possible types of close-packed rhombohedral crystals, but the Sm-type structure is the only one that occurs in pure elements, so I will use Sm-type and CPR interchangeably. This structure may also be called 9R, because it has 9 close-packed layers per rhomohedral unit cell.
| Alternative Names |
| 9R |
| Sm-type (Samarium-type) |
| Close-Packed Rhombohedral (CPR) |
Sm-type is an advanced crystal structure (because it doesn’t correlate 1-1 to a Bravais lattice), so if you’re searching for information about it, I’m assuming you’re a somewhat advanced student in materials science.I’ll give you the facts quickly and with little explanation, but if you are new to materials science and don’t know what something means, you can check out my articles on the FCC, BCC, or HCP crystals, which explain terminology more slowly.
The Sm-type crystal structure is close-packed with rhombohedral symmetry, with 9-layer stacking order ABCBCACAB-ABCBCACAB, in contrast with FCC (ABC-ABC), HCP (AB-AB) and DHCP (ABAC-ABAC). The close-packed rhombohedral structure belongs to space group #166 or R-3m, Strukturbericht C19, and Pearson symbol hR3. α-Sm is the prototype.
Although this crystal structure is rarely discussed in materials science classes, a surprising amount of elements take this form at non-STP conditions.
Outline
Common Examples of Close-Packed Rhombohedral Materials
At standard temperature and pressure (STP), samarium takes this 9R crystal structure.
However, in non-STP conditions, lithium, gadolinium, terbium, holmium, erbium, thulium, and lutetium display the Sm-type crystal structure.
Close-Packed Rhombohedral Coordination Number
Since the Sm-type crystal is close-packed, it must have 12 nearest neighbors (NNs) in the ideal case, so coordination number CN=12.
Close-Packed Rhombohedral Lattice Constants
Rhombohedral crystals have 3 lattice parameters: a, b, and c. In this particular rhombohedral crystal, lattice parameters a and b have the same length, and are at 120º to each other. While each individual layer has 6-fold rotational symmetry, it’s rhombohedral instead of hexagonal because the combination of layers stacked on each other does not have 6-fold symmetry.
Thus, we are left with a and c (or the conventional a and c/a ratio).
is just the distance between two atoms in the close-packed direction, and since they touch, that distance is
.
Calculating c is a bit trickier, but if you already know how to calculate the c/a ratio for HCP, then this is easy. I’ll re-use my graphic from my HCP article:
Although the stacking patterns are different, both HCP and Sm-type share the same first two layers, so we’ll start by finding the height difference between two layers. The atoms are touching in the ideal hard sphere model, which is a distance of or
.
The value between two layers is labeled as “” in the image above. If you follow the trigonometry, that results in
. Remember though, this “
” is the HCP’s lattice parameter c, which is the vertical distance between two close-packed layers.
For the Sm-type lattice parameter , there are 9 layers, so this distance repeats 9 times. In other words, the Sm-type lattice parameter is exactly 4.5 times the size of the HCP lattice parameter.
Thus, the Sm-type c/a ratio is , and because
, then
.
These are theoretical values, however, calculated from the hard sphere model. Structures usually take these lower-symmetry close-packed arrangements (instead of FCC or HCP) because they have some slight asymmetry in the c-direction, so the c/a ratio is usually not ideal.
If you wanted to describe the Sm-type crystal with math, you would describe the cell with the primitive lattice vectors:
And basis vectors (Lattice coordinates and Cartesian coordinates, respectively):
Close-Packed Rhombohedral Atomic Packing Factor
Yep, all close-packed structures have an APF=74% in the ideal case, but what if the crystal is not ideal? If you know the lattice parameter a and c/a ratio, you can still calculate the APF even in a non-ideal case.
First, you need to calculate the volume of the unit cell.
The hexagon is 6 equilateral triangles, with side length a. Using the proper formula (or clever trigonometry), you’d find that the area of an equilateral triangle is . Six of those gives us our hexagon, and the height is c.
Working this out, we have , for the volume of the hexagonal prism in terms of a and c/a.
Now we need to determine the volume taken up by atoms.
Sm-type close-packed rhombohedral has 12 vertex atoms which each contribute ⅙ of their total volume, for a total of 2 whole atoms. There are 2 face atoms which each contribute ½ of their total volume, for 1 whole atom. There are 12 edge atoms, which each contribute ⅓ of their total volume, for 4 whole atoms. There are 20 atoms completely contained within the unit cell, which each contribute their full volume.
Adding up those atoms gives 27 atoms per unit cell. Modeling each atom as a sphere, the volume of each atom is .
Thus, the APF for Sm-type close-packed rhombohedral is
Where a and c/a are the lattice parameters, and is the atomic radius.
Assuming the ideal case of and
, then
So we arrive at the expected result, 74% packing.
Final Thoughts
The Sm-type, Close-Packed Rhombohedral, 9R crystal structure does not appear in very common materials, but it does show up in many rare elements at non-STP conditions.
This crystal structure is close-packed with rhombohedral symmetry, and is composed of 9 close-packed layers with stacking order ABCBCACAB-ABCBCACAB.
Like all close-packed structures, Sm-type has theoretical APF=74% and CN=12.
It is worth noting that while this page listed several theoretical values for abstract Sm-types crystals, real Sm-types crystals vary in their c/a ratios.
References and Further Reading
If you want to know more about the basics of crystallography, check out this article.
If you weren’t sure about the difference between crystal structure and Bravais lattice, check out this article.
I also mentioned atomic packing factor (APF) earlier in this article. This is an important concept in your introductory materials science class, so if you want a full explanation of APF, check out this page.
If you’re interested in advanced crystallography or crystallography databases, you may want to check out the AFLOW crystallographic library.
For a great reference for all crystal structures, check out “The AFLOW Library of Crystallographic Prototypes.”
Single-Element Crystal Structures and the 14 Bravais Lattices
If you want to learn about specific crystal structures, here is a list of my articles about Bravais lattices and some related crystal structures for pure elements.
1. Simple Cubic
2. Face-Centered Cubic
2a. Diamond Cubic
3. Body-Centered Cubic
4. Simple Hexagonal
4a. Hexagonal Close-Packed
4b. Double Hexagonal Close-Packed (La-type)
5. Rhombohedral
5a. Rhombohedral Close-Packed (Sm-type)
6. Simple Tetragonal
7. Body-Centered Tetragonal
7a. Diamond Tetragonal (White Tin)
8. Simple Orthorhombic
9. Base-Centered Orthorhombic
10. Face-Centered Orthorhombic
11. Body-Centered Orthorhombic
12. Simple Monoclinic
13. Base-Centered Monoclinic
14. Triclinic
Other articles in my crystallography series include:
Introduction to Bravais Lattices
What is the Difference Between “Crystal Structure” and “Bravais Lattice”
Atomic Packing Factor
How to Read Miller Indices
How to Read Hexagonal Miller-Bravais Indices
Close-Packed Crystals and Stacking Order
Interstitial Sites
Primitive Cells
How to Read Crystallography Notation
What are Point Groups
List of Point Groups
What are Space Groups
List of Space Groups
The 7 Crystal Systems