
Okay, so I titled this “Allotropes vs Isotopes: All Differences” because I thought that would make this come up in google searches, but there’s really only one difference. Or maybe, there’s only one similarity?
When I told my wife Ewelina that English-speakers often confuse “allotrope” with “isotope,” she became confused. These words actually mean very different things–they just sound similar.
Allotropes and isotopes work on different length scales. An allotrope is one way that atoms can be arranged in a solid. An isotope is one way that protons and neutrons can form the same atomic element.
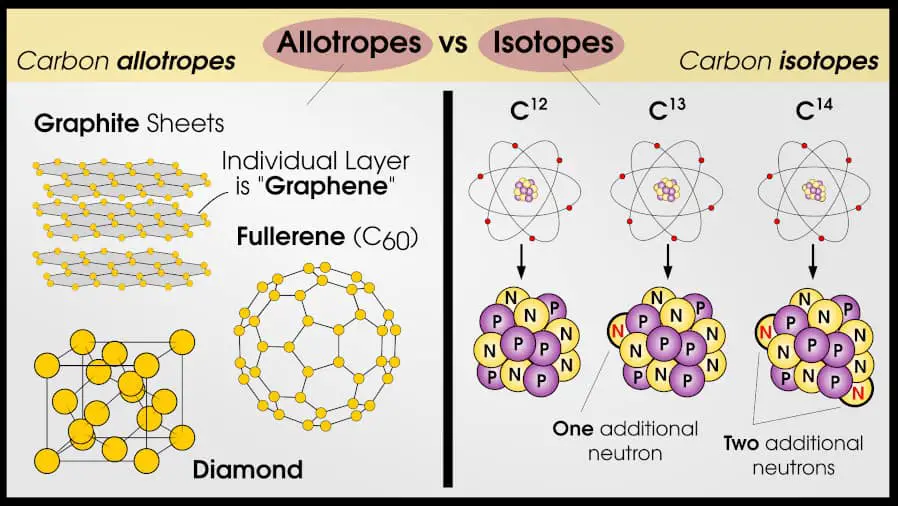
Isotopes are atoms that have a different number of neutrons than a different isotope. Allotropes are crystals that have a different atomic structure than a different allotrope.
You can see that allotropes and isotopes are completely different and really have no relationship to each other at all. But if you were confused about their difference, you may not have learned much about isotopes and allotropes. Let’s dive into each one individually.
Outline
What are Isotopes?
Each element on the periodic table is defined by the number of protons it has. For example, carbon has atomic number 6, so it has 6 protons. Any atom with 6 protons is a carbon atom.
Atoms are composed of protons, electrons, and neutrons. In most cases, atoms will be electrically neutral, so there will be enough electrons to match the protons (6, for carbon). At first glance, you might think there could be any number of neutrons to match the protons. Or perhaps you might think there should be some rule, like 1 neutron per proton.
The actual answer is somewhere in the middle. Every element can have multiple versions with different numbers of neutrons (that’s an isotope!).
However, some isotopes will be more stable than others. Carbon, for example, can occur with 6 protons and 6, 7, or 8 neutrons. The “regular” one is Carbon-12, which means it has 6 neutrons.
When naming isotopes, we use the atomic weight. Protons and neutrons have almost exactly the same mass, and electrons have so little mass that you wouldn’t notice it. The atomic weight is just the number of protons plus the number of neutrons.
Carbon always has 6 protons, so 6 more neutrons and it will be Carbon-12. Carbon-13 has 7 neutrons, and Carbon-14 has 8 neutrons. (There is also Carbon-8 to Carbon-22, but these can only be made in a laboratory and they are very unstable).
Some isotopes will be more stable than others. In carbon, carbon-12 is much more stable and abundant than carbon-13 or carbon-14. Carbon-14 is unstable but has a very long half-life (more on this in the next section).
Isotopes are the reason that the atomic weights on the periodic table are not nice whole numbers. Carbon-12 weighs exactly 12 amu, carbon-13 weighs 13 amu, etc. Since the periodic table says that carbon’s atomic weight is 12.011 amu, that means that the average weight is 12.011 amu. In other words, almost all carbon is carbon-12, but there is enough carbon-13 and carbon-14 to increase the average weight of carbon to 12.011 grams per mole.
Similarly, hydrogen has an atomic weight of 1.008 amu. Most hydrogen is hydrogen-1 (protium), but there are small amounts of hydrogen-2 (deuterium) and hydrogen-3 (tritium). These isotopes of hydrogen get fancy names because they each have unique applications.
Why do Isotopes Matter?
In general, stable isotopes behave pretty much the same. Hydrogen-1 and hydrogen-2 have very similar chemical properties, because the extra neutron doesn’t change much outside of the nuclear forces within the atom.
Tracer Isotopes
One use for similar-behaving isotopes is as a tracer. Around the globe, different elemental isotopes occur in slightly different ratios. This information is a great tool for assessing environmental impact like water flow, or solving geological puzzles about the planet before human record.
Different isotopes can be placed in the body along with pharmaceuticals to track the drug movement. Since the rare isotope doesn’t behave differently than the common isotope, it’s more accurate to track an isotope of the same material rather than adding a new molecule which might behave differently than the drug in question.
Radioactive Decay
Some isotopes are radioactive, which means that the isotope is not stable. To achieve a more stable configuration, these atoms may shed some number of protons and neutrons. This can let us change lead into gold!
Yes, changing lead into gold has actually been accomplished by scientists.If you make a radioactive lead isotope that wants to eject 3 protons, you’ll get gold! Unfortunately, making this lead isotope and then converting it into a gold isotope is extremely expensive. It’s better just to buy gold outright 🙂
Carbon Dating
Another advantage of isotope’s “radioactive decay” is that it is extremely predictable and can be modeled exponentially by a single term: half-life. Half-life is the amount of time it takes a radioactive isotope to decay into a different isotope or element. If you started with 100 grams of something that had a half life of 10 minutes, then every 10 minutes you would have half of what you started with. After 10 minutes you would have 50 grams, after 10 more minutes you would have 25 grams, ater 10 more minutes you would have 12.5 grams, and so on . . .
Have you heard of carbon dating? It is a technique used to evaluate how old something is. Carbon dating works by measuring the ratio of carbon-14 to carbon-12. Since carbon-14 is radioactive but has a slow half life (5,730 years), you can measure the concentration of carbon-14, calculate how long it has been decaying, and approximate the age of the object.
Now that you thoroughly understand isotopes, let’s move to that other, barely-related topic: allotropes.
What are Allotropes?
Unlike isotopes, allotropes don’t care about neutrons at all. For an allotrope, we assume it has the regular isotope of that element–the same assumption we make for all non-nuclear chemistry.
With an allotrope, we think about the way those atoms can be arranged. You may have heard of carbon allotropes, so I’ll start with something you may not have heard of: crystals
Unlike liquids and gases, in which atoms are arranged randomly and constantly moving around, most solids exist as crystals. That means the atoms are arranged in a particular repeating order, and they mostly stay in the same place.
Here, let’s take a look at the real phase diagram of water.
You see all those roman numerals in the blue area? Those are each a different crystal arrangement of ice (technically ice is a polymorph instead of an allotrope, but these words mean the same thing except allotrope only applies to pure elements). In chemistry class you probably just labelled the whole region “solid” because chemists are afraid of solids!
However, each of these crystal arrangements are important. As the element changes from one solid crystal to another, it may change density, magnetism, strength, and more.
For example, let’s look at carbon allotropes. The 3 main allotropes of carbon are diamond, graphite, and buckminsterfullerene.
Why do Allotropes Matter?
Carbon
Between these carbon allotropes, the different atomic arrangements lead to very different properties.
Buckyballs can be expanded into nanotubes, diamond is the strongest naturally-occurring material, and graphite is relatively soft and used as a lubricant.
Did you know that if you put a diamond in the oven, it will turn into graphite? That’s polymorphism (or allotropy) at work.
Thermodynamically, graphite could arrange itself in a diamond structure or a graphite structure. As it turns out, graphite is the more stable form. If you give diamond enough thermal energy for the atoms to make their own decisions, they’ll choose to form graphite.
Iron
Or, consider iron. Iron can be allotropic. Just look at the phase diagram below! (Phases are states of matter + solid polymorphs/allotropes) This graph tells you the most stable allotrope of iron at a particular temperature and pressure. Each of those greek symbols is a different crystal structure.
Let’s focus on reasonable pressures and temperatures (let’s say atmospheric pressure and up to 1500°C). The critical allotropes are ⍺ and γ.
Suppose you took a wire of iron and heated it up. At first, the length of the wire would expand due to thermal expansion. But at about 1390°C, when the ⍺ iron turns into γ iron, the wire would dramatically shrink because γ is a more dense way to pack atoms than ⍺.
Additionally, at the moment when the structure becomes γ instead of ⍺, the iron would lose its ferromagnetism. In γ, the iron atoms are arranged in a way that inhibits ferromagnetism.
Tin
Did you know that Napoleon failed his invasion of Russia because of polymorphism? (According to one of my old professors.)
It turns out that the jackets on Napoleon’s army had tin buttons. At regular temperatures and pressures, tin is a ductile metal with a body-centered tetragonal structure. At cold temperatures, however, tin turns into the same crystal structure as diamond.
Unfortunately, diamond cubic is a much less-dense way to pack atoms, so there is a huge volume expansion when tin cools down. Tin can’t survive the stress from this large volume change so it basically disintegrates.
When Napoleon invaded Russia, the soldiers’ tin buttons disintegrated in the cold.They had to survive the Russian winter with open jackets!
Allotropy (and phase transformation in general) is a very important tool/consideration when engineering materials.
Final Thoughts
Hopefully, I’ve really hammered home the idea that “allotrope” and “isotope” are only similar in the way the words sound.
Isotopes are an alternate form of the atom which has a different number of neutrons.
Allotropes are an alternate form of the crystal which has a different packing of atoms.
Isotopes are important in nuclear chemistry and physics, with applications ranging from smoke detectors, forensic geology, and nuclear fusion.
Allotropes are important in materials science and engineering to consider the ways that an element can be arranged so you have the most useful allotrope and don’t accidentally transform into a “bad” allotrope.
| Allotropes | Isotopes |
| Allotropes are crystals that have a different atomic structure than a different allotrope | Isotopes are atoms that have a different number of neutrons than a different isotope |
| An allotrope is one way that atoms can be arranged in a solid | An isotope is one way that protons and neutrons can form the same atomic element |
| Carbon allotropes: graphite, diamond, fullerene, graphene | Carbon isotopes: C12, C13, C14 |
| Allotropes play an important role in materials science and engineering to consider the ways that an element can be arranged so you have the most useful allotrope and don’t accidentally transform into a “bad” allotrope | Isotopes play an important role in nuclear chemistry and physics, with applications ranging from smoke detectors, forensic geology, and nuclear fusion |
References and Further Reading
If you want to know the differences between allotropy and polymorphism, check out this post!
Are you curious about the differences between the two most common crystal structures of metals? You’ll find out here!