
When I was an undergraduate student, I never liked superalloys–I always thought it was pretentious, naming them “super.” Now that I’m doing my PhD on superalloys, however, I have come to appreciate just how unique this alloy system is.
Superalloys are metal alloys that can operate at a high fraction of their melting point. Superalloys must have high strength, high melting points, resistance to corrosion or oxidation, and creep resistance.
That’s the best definition of a superalloy I can give you, although the term is a bit nebulous. For a full breakdown of the etymology of superalloys (and advanced metallurgical concepts), you can check out my article Advanced Metallurgy of Superalloys.
In this article, I will mostly focus on the most common type of superalloy, the nickel-based superalloy with a gamma-prime microstructure. This article is a basic introduction to superalloys and I’ll explain relevant metallurgy concepts along the way.
Outline
Why are Superalloys Important?
In a word: efficiency. (Also, creep)
Superalloys are the materials best-suited for practical high temperature performance. Yes, there are materials that can survive hotter temperatures (like tungsten, or ceramics), but those can only work in special situations.
Ceramics are brittle; tungsten will oxidize. These materials have their role in crucibles or welding electrodes, but if you want to build a machine that has to survive high temperatures, superalloys have the best combination of strength, oxidation resistance, creep resistance, and more (at high temperatures).
And high temperatures are a bigger issue than most people realize. If you want something to go faster, it also gets hotter. For example, the SR-71 blackbird is not airtight. Well, not airtight at room temperature.
When the plane travels fast, air resistance causes the ship to heat up, and the outside layer expands. If the blackbird was airtight at room temperature, thermal expansion would cause cracking at maximum speed. The solution is to give the plane holes that will seal due to thermal expansion, and heat the ship at room temperature by other means.

In many engineering applications, temperature is the barrier that needs to be crossed. Engineers know how to make faster planes or cars. The problem is that we don’t have materials that can survive the temperatures necessary.
Superalloys are used in the most important temperature-limited applications. Specifically, they are usually used for turbine blades (more on this in the applications section).
If you learned about Carnot efficiency in high school, you may recall that there is a thermodynamic rule that says energy efficiency is related to temperature difference. In other words, if you burn natural gas to convert it to electricity, the temperature at which you burn the gas will determine how much energy is lost. Higher temperatures means less energy lost.
Considering the amount of energy humanity uses, a 1% increase in efficiency is worth billions of dollars. The part of the power plant that limits temperature is the turbine blade. Engineers devote their careers to improving the material of the turbine blade–and the result is a superalloy.
Even if we stopped burning fossil fuels for electricity, superalloys will still be vital to transportation. In almost every sector of technology, scientists have identified a path forward that uses renewable energy. Solar panels can generate electricity, and cars and then run on that electricity. Stoves and heating units in peoples homes are already in transition to electric, instead of gas.
However, there is one technology that can’t be replaced by electricity: jet airplanes. Jet turbines work on the same principle as gas turbines–converting heat into thrust. Better superalloys means that the turbine can run at a higher temperature, allowing the airplane to travel farther, faster, and on less fuel.
Superalloys are an expensive alloy system, but it is critically important for high-temperature applications. Modern power plants and air transportation would not be possible without superalloys.
Before I move on, I want to address one final point: creep. Creep is how a material deforms at high temperatures and long times (creep is how glaciers move). When designing high-temperature alloys, usually creep is the critical property.
You might have a stronger, higher-melting point alloy than a superalloy, but it would not be usable over a long period of time. At these high temperatures, materials don’t fail because they experienced too much force or too high temperature; engineers are smart enough to avoid that in almost every situation. The materials actually fail because, over hundreds of hours of use, the material creeps and creeps and creeps, microns at a time, until it is no longer usable.
Why are Superalloys so Special?
Well, if superalloys are so important to technology, they must have some unique property! What is it?
Unfortunately, the definition of superalloys fails a bit here. For the sake of simplicity, every time I say “superalloy” in this article, you can assume I mean “nickel-based superalloy.”
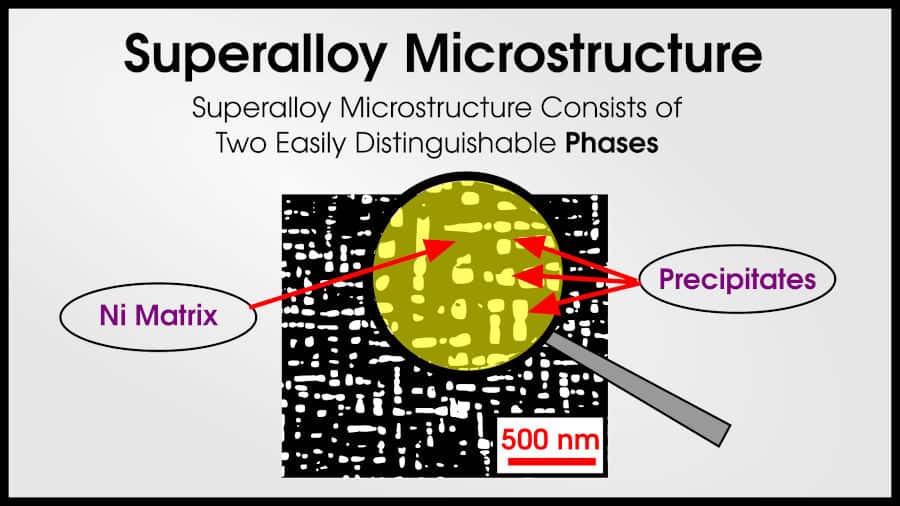
Nickel-based superalloys have an amazing microstructure that gives the material excellent high-temperature properties, but there are other alloy systems that are called “superalloys” simply because they have good high-temperature properties (without the special microstructure).
Before I explain this microstructure, however, I want to show you a graph of yield strength vs temperature.
Yield strength is the most basic measurement of strength that engineers use (you can check out this article if you want a deeper explanation).
As you can probably guess, as temperature increases, strength should decrease. Thermal energy means atoms vibrate faster, so they are more likely to slip past each other. In almost all materials, increasing temperature decreases strength.
Nickel-based superalloys are the exception, as you saw in the graph. As temperature increases, there is a “hump” of increased strength. This is called anomalous yield strength because the strength doesn’t behave normally.
The anomalous yield strength means that–at a high fraction of its melting point–nickel-based superalloys have a much higher strength than most materials. That’s why they are so good at high temperatures.
Nickel-based superalloys have an anomalous yield strength because of their unique γ/γ’ microstructure (pronounced gamma, gamma prime).
The reason that the microstructure does this is pretty complicated, and hard to understand without a background in materials science. I’ll walk you through the full explanation in another article. I was frustrated that I never learned this in undergrad, so if you want me to use words you don’t know right now, the anomalous yield strength is because of partial dislocation cross-slip locking.
But enough about that–what is gamma and gamma prime?
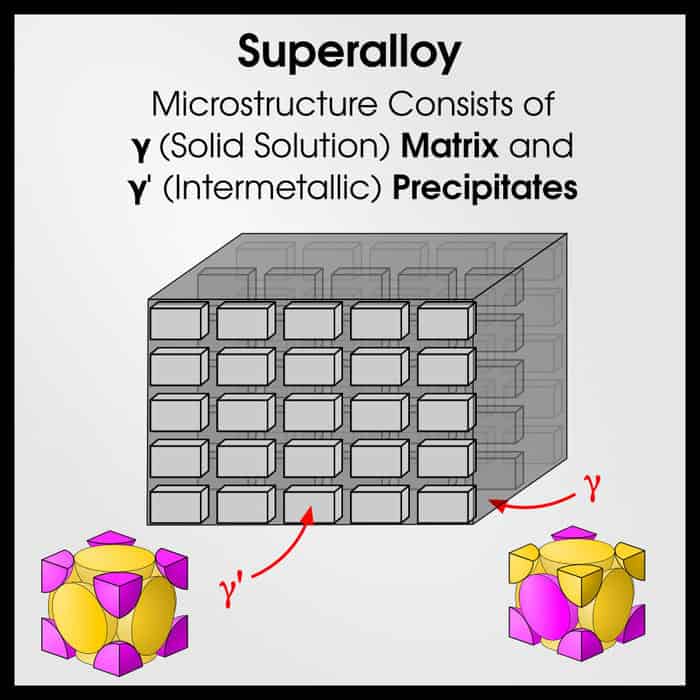
Gamma is the 3rd letter in the Greek alphabet, γ. For whatever reason, this has historically been the letter that refers to an FCC phase. γ is also used to refer to austenite in steel (because austenite is FCC).
FCC means “Face-Centered Cubic.” It is one of the most common–and most stable–crystal structures. To understand what the FCC crystal looks like, imagine a cube with an atom on each corner of the cube, and each face of the cube. That’s what FCC is!
γ’ is an intermetallic compound, with L12 structure. This is an ordered FCC arrangement.
γ’ has Ni atoms on the faces and a different kind of atom on the corners. Usually, this other atom is Al.
Let’s look at a mixture of 75% Ni 25% Al to illustrate the difference between γ and γ’.
γ is an FCC substitutional solution. Pure Ni would be a regular FCC, as you saw previously. However, Al can dissolve in Ni. Supposing that 25% Al could dissolve in Ni, you would get something like the image on the left. ¼ of the Ni atoms would be randomly replaced by Al (if you aren’t sure how I counted atoms to see that ¼ of them are Al, I recommend you read this article).
In γ’, the structure has an order. The Al atoms always sit on the corner, and Ni will sit on the faces. It’s possible that γ’ could have substitution as well–there is not some law preventing Ni from going to the corners, but the image on the right shows the preferred state. If there is preference for the atoms to order like this, the phase is L12. If there is no preference, the phase is just FCC.
It’s difficult to explain much about γ’ without explaining dislocations, but I’ll do my best. The combination of γ and γ’ is great because γ is tough and holds the hard γ’ together. The alloy benefits from strength and toughness because of the precipitate/matrix relationship.
This kind of strengthening is called precipitate strengthening and is common in most advanced alloys.
γ’ is additionally special because it is coherent.
It turns out that the distance between atoms in Ni and Ni3Al is not very large. Ni3Al might be 1% smaller than the rest of the γ lattice. As a result, the interface between γ’ and γ is connected. This is not true in incoherent precipitates (more common).
If you imagine that a row of atoms needs to slide past another row, the connected interface between γ and γ’ allows this to be possible. This is what makes γ’ precipitates tougher than most other kinds of precipitates. There is lattice strain so sliding the atoms past each other is not easy, but it is possible.
With incoherent precipitates, the difference between the matrix and precipitate is abrupt. There is no way to push a row of atoms in the matrix, because they wouldn’t connect to the precipitate. If enough force tried to push a row of atoms past each other, the material may just develop a crack.
In addition to precipitate strengthening, superalloys benefit from solid solution strengthening. This kind of strengthening is present in all alloys, but it is especially noteworthy in superalloys because these alloys are so well-developed that a commercial superalloy may have a dozen elements in it.
History of Superalloy Development
According to this paper, the United States became interested in gas turbine development around 1905. The metals for these turbine blades were made of the three intersoluble FCC metals: Fe, Ni, and Co.
From 1910-1915, austenitic (γ phase) stainless steels were developed for the high temperatures in gas turbines. By 1929, 80Ni-20Cr alloy was the norm, with small additions of Ti and Al. Although early metallurgists did not know it yet, they were forming small γ’ precipitates in Ni-based superalloys. These alloys quickly surpassed Fe- and Co-based superalloys, which were strengthened by carbides and solid solution strengthening.
Although Cr was great for protecting the alloys from oxidation and corrosion up to 700°C, metallurgists began decreasing Cr in favor of Al, which had oxidation resistance (but not corrosion resistance!) at much higher temperatures. The lack of Cr caused issues with hot corrosion, so coatings needed to be developed.
Around 1950, vacuum melting became commercialized, which allowed metallurgists to create higher purity alloys with more precise composition. Vacuum arc remelting is a process for creating precise alloy compositions, and it’s possible to do on a small scale.
The technique is essentially welding in a vacuum. Scientists take pure elements and combine them in a metal, water-cooled crucible. The crucible is sealed in a vacuum chamber with an electrode on top. The electrode–just like in welding–sparks an arc to the raw metals.
This arc is hot enough to melt any material, but since the crucible is water-cooled, only the raw metals are melted. By flipping the sample and melting it several times (that’s why it’s called “remelting”), you can create a homogenous alloy even on a small scale. The vacuum and high temperatures maintain the sample’s purity, avoiding oxides and ensuring complete mixing of the elements.
In the 60s and 70s, metallurgists changed focus from alloy chemistry to alloy processing. Directional solidification was developed to allow columnar or even single-crystal turbine blades. Oxide dispersion strengthening could obtain very fine grains and superplasticity. I’ll talk more about directional solidification in the next section.
In modern times, there are six generations of Ni-based superalloys, strengthened by phases beyond γ’. One large focus in recent commercial alloys has been rhenium, which dramatically increases creep resistance for reasons that scientists still argue about (we’re pretty sure it has something to do with rhenium’s slow diffusivity in nickel).
Nickel-based superalloys, however, have pretty much reached their peak development. Most improvements nowadays come from thermal barrier coatings–or other alloy systems.
Although the γ/γ’ microstructure is so far only used in Ni, γ’ has recently been discovered in cobalt. (Well . . . this is a long story. Technically it was discovered in 1971, but that person never published it . . . ). Cobalt has a higher melting point than nickel (as well as other potential advantages) so perhaps cobalt-based superalloys are the future.
Only time will tell.
How are Superalloys Made (Processing)?
Industrial-scale superalloys are typically made by one of two processing methods: cast superalloys and wrought superalloys. There is also powder metallurgy or additive manufacturing (3D printing) of superalloys, but this processing method is still in the developmental stage.
Wrought Superalloys
“Wrought” basically means “forged.” Wrought superalloys are cast into long rectangles called billets, then heated, deformed, and forged into the final shape.
Forging the superalloys can lead to a generally more-homogenous mixture than direct casting, but the method can’t be used in all alloys. Wrought alloys are typically more ductile than cast alloys, and have grain boundaries and work hardening.
Wrought superalloys today are usually used in large disks.
Cast Superalloys
Cast superalloys are directly solidified into shape.
Some alloys can only be cast, not forged (if ductility is not high enough). However, cast alloys may have element segregation, large grains, and dendrites.
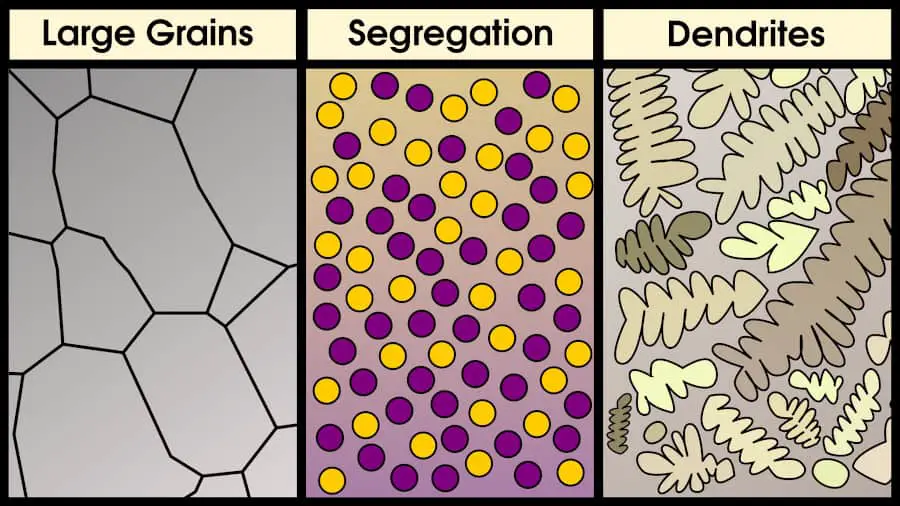
However, cast superalloys are more common today in turbine blades, for one simple reason: with casting, it’s possible to create single crystal turbine blades.
Superalloy Grain Size
In most applications, small grain size is good. However, grain boundaries are bad for creep. Since creep is the most important property for superalloys, larger grains–or better yet, a single crystal with only one grain–is ideal.
Don’t know what grains are? Click here to expand.
Grains are groups of atoms in one crystallographic direction. Most people think of diamond as a crystal and are incredulous when I tell them that steel is also a crystal. The difference is that diamond (at least the kind you buy for a ring) is a single crystal, which means it has no grains. Every atom in the diamond repeats exactly like every other atom. Most materials are polycrystalline, which means they have many grains. Each grain has atoms all arranged in the same direction, but grains are oriented randomly compared to each other. Grains are also super small, which is why you don’t notice any “crystals” in steel.
Superalloys can have 3 types of grain patterns: equiaxed polycrystalline, columnar polycrystalline, and single crystal.
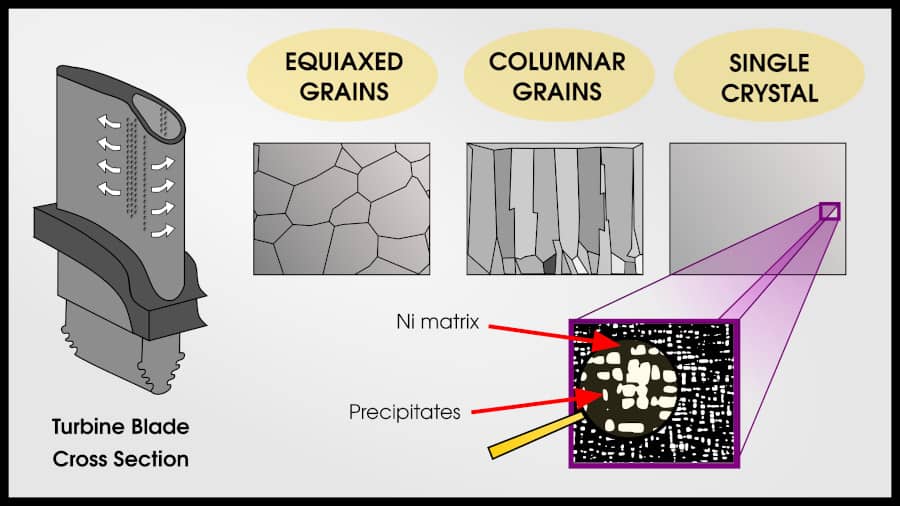
Equiaxed grains are the “default” pattern for metals. Each grain is roughly the same size and shape. Most methods of solidifying from liquid will result in an equiaxed polycrystal.
Columnar grains are more creep-resistant than equiaxed grains. In the direction of stress, there are no grain boundaries. Columnar grains are created by slowly changing the temperature gradient so that an initial set of polycrystalline grains forms, but then the liquid on top of these grains solidify in the same pattern.
The end result is that same number of grains as the initial nucleation, but each grain travels the whole length of the turbine blade.
A single crystal turbine blade has the best creep properties for three reasons:
- There are no grain boundaries at all, which is good for creep
- Sometimes “bad” phases precipitate at the grain boundaries, so removing grain boundaries avoids these phases
- The crystal can be oriented so that the maximum strength is in the direction of maximum stress.
When you have a crystal, the strength of the crystal is not uniform. Force at a 90 degree angle will not necessarily produce the same displacement as force at a 45 degree angle.
However, most metals you see in real life are polycrystalline. When you average thousands or millions of grains, the end result has an average strength in all directions.
With a single crystal, there is no average. If the turbine blade will only experience force from one direction, you can orient the crystal so that the strongest crystal direction is in the same direction as the force.
Single crystal alloys can be produced by a few different ways, although they are all expensive. One popular method of making single crystal turbine blades is to “seed” an initial grain by using a spiral pattern.
At first, several grains nucleate. But as they grow through the spiral pattern, eventually only one grain remains. If engineers can cool the rest of the turbine blade very slowly, so no other grains nucleate, the entire blade will be a single crystal!
Thermal Barrier Coatings
The final part of a superalloy isn’t an alloy at all, but a coating. I’ve written an entire article on thermal barrier coatings so if you’re really interested in them, I suggest reading that.
In short, thermal barrier coatings are a coating applied to superalloys that protect the superalloy from high temperatures. If the superalloy’s maximum operating temperature is 1000°C but the thermal barrier coating can protect it from 200°C, then the gas turbine could be run at 1200°C.
Thermal barrier coatings consist of a
- Topcoat designed for thermal insulation
- Bond coat designed to prevent oxidation and keep the topcoat and superalloy from diffusing into each other, and
- Thermally grown oxide designed to hold the topcoat and bond coat together, and additionally help keep oxygen away from the superalloy
My wife did a bit of undergraduate research on thermal barrier coatings, so if you’re interested I have an in-depth article here.
Superalloy Applications
Superalloys are the pinnacle of modern metallurgy. They combine over a dozen elements, many of them rare, in exact quantities. The alloys are often processed as single crystals, and undergo precise heat treatments to precipitate the optimal phases.
That is to say: superalloys are expensive. The main competitor of superalloys is steel, which can be thousands of times cheaper than a fully-engineered superalloy. Superalloys are also denser than steel (and especially lightweight metals like titanium and aluminum).
In most cases, the cost of superalloys is not worth their superior properties (especially since these properties are only superior at high temperatures).
If you’ve wondered why all my examples so far have been about superalloys in the context of a gas turbine, that’s because gas turbines are the main application where the cost of superalloys is paid for by their superior properties.
Gas turbines and airplanes and powerplants function roughly the same, and superalloys are used to make many parts of the turbine.
Most people think of superalloys being used in turbine blades. These are the part of the turbine that spins in the presence of hot gas. The turbine blades need to be very creep resistant to avoid the centripetal force. These are usually made of a single crystal Ni-based superalloy.
Superalloys are also used in the turbine disc. The disk connects the rotary shaft to the blades. The disc needs to be strong, but since it experiences a lower temperature than the blades, turbine discs are usually polycrystalline. They are often made of Fe- or Co-based superalloys, which can be stronger and more fatigue-resistant at lower temperatures.
Turbochargers may also be made of superalloys. Since these are driven by exhaust gases, they need to be corrosion/oxidation resistant, and strong at high temperatures. Turbochargers have much smaller blades than turbine blades, so creep is a lower priority.
Airfoils/Vanes/Nozzles have similar requirements as turbochargers. Vanes accelerate hot gas flow. These are usually made of Co-based superalloys because they don’t need to be very strong, but they do need to be temperature- and corrosion-resistant, and good weldability is a plus.
Superalloys may also be used in very hot sections of nuclear reactors or automobile engines, or in any situation where creep resistance warrants a more expensive alloy than steel, but these are niche uses. Most superalloys built on this planet will be used in a gas turbine.
From this paper, superalloys (which also includes Co-based superalloys and high-temperature steels) are used in
| Main Category | Examples |
| Aircraft gas turbines | disks, combustion chambers, bolts, casings, shafts, exhaust systems, cases, blades,vanes, burner cans, afterburners, thrust reversers |
| Steam turbine power plants | bolts, blades, stack gas, re-heaters |
| Reciprocating engines | turbochargers, exhaust valves, hot plugs, valve seat inserts |
| Metal processing | hot-work tools and dies, casting dies |
| Medical applications | dentistry uses,prosthetic devices |
| Space vehicles | aerodynamically heated skins, rocket engine parts |
| Heat-treating equipment | trays, fixtures, conveyor belts, baskets, fans, furnace mufflers |
| Nuclear power systems | control rod drive mechanisms, valve stems, springs, ducting |
| Chemical and petrochemical industries | bolts, fans, valves, reaction vessels, piping, pumps |
| Pollution control equipment | scrubbers |
| Metals processing mills | ovens, afterburners, exhaust fans |
| Coal gasification and liquefaction systems | heat exchangers, re-heaters, piping |
Fe- and Co-based Superalloys
Fe-based superalloys are just steels. But I guess they are a specific kind of steel, used for high temperature applications. Steel is cheap and very strong, so it is the alloy family of choice for most engineering applications. For example, steam turbine blades–which don’t get as hot as gas turbine blades–are often made of steel (or “iron-based superalloy) because it’s cheaper.
Co-based superalloys (before the γ’ was found) are not as strong as Ni-based superalloys, but can be used at higher maximum temperatures. These alloys were typically used in very high temperature applications that didn’t have as much stress. Additionally, Co-based superalloys are a bit more weldable than Ni-based superalloys, so they may be used in applications that required on-site welding such as vanes
Final Thoughts
Superalloys are complex! They are among the most-developed alloy systems because they are critically important to power generation and transportation.
Nickel-based superalloys have extreme creep resistance and an anomalous yield strength because of their microstructure of γ matrix and γ’ precipitates.
This article covered the basics of superalloys: their history, applications, and simple metallurgy. If you’re looking for a more advanced article that talks about more phases like TCPs and γ’, strengthening mechanisms, and creep theory, you will soon find that article on our website!
References and Further Reading
For a full historical account of superalloy development, look no further than this paper by Chester Sims.
If you want to know more about the parts of gas turbines–and what requirements each part needs to have– you should read this paper by Dr. Henry Bernstein.
If you would like to see compositions and properties of current commercial nickel-based superalloys, you can check out this page from StarDustImpex.
This page was our source for superalloys applications in gas turbines.