
When I was a teaching assistant for an introductory materials science class, the first question students asked me was about the difference between a metal alloy and a composite. They wanted to know if alloys were a subset of composites.
Alloys and composites are separate concepts. One is not a subset of the other.
An alloy is a combination of elements (at least 1 metal) in solid-solution with overall metallic properties. Steel is an alloy of iron and carbon.
A composite is a combination of other materials, where the mixed materials remain physically distinct. Reinforced concrete is a composite of steel, cement, and gravel.
A compound is a combination of elements (usually 2) with chemical bonds, so the compound has no relationship with its base elements. Sodium Chloride (table salt) is a compound of sodium (an explosive metal) and chlorine (a toxic gas).
If you already know the difference between alloys and composites and you want clarification about the “gray area,” go ahead and skip this next section. For the rest of you, let me explain the differences between alloys, composites, and compounds using basic chemistry.
Outline
A Review of Basic Chemistry
Click here to expand
In chemistry, you probably learned about chemical bonds, solutions, and mixtures.
A chemical bond occurs when two elements come together and their electrons interact. This interaction can dramatically change the properties of the two atoms. Since it makes more sense to consider the new material as one “thing,” we call it a compound. Compounds are made of more than 1 element, chemically bonded into a single phase.
For more on physical vs chemical bonds, the preview for this paper has a nice table.
I now want to introduce the idea of phases. The idea that a material is all “one thing” is the same as saying it is “one phase.” A phase is any portion of matter that shares all of the same properties. For example, if you selected some atoms of water, you would not be able to tell the difference between water somewhere else.
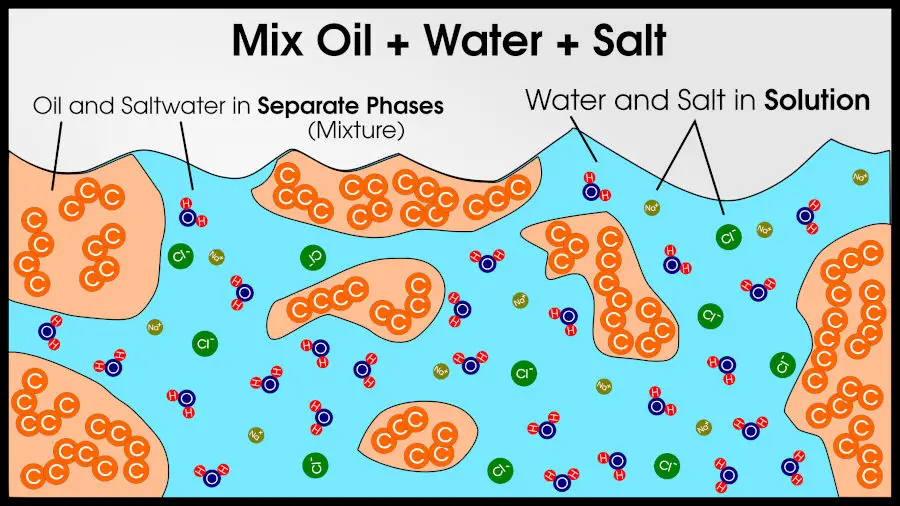
If you added salt, the salt atoms would spread out evenly through the water. Before the salt is added to water, it is clearly a different phase. Table salt is a solid, crystalline arrangement of sodium and chlorine atoms. But once you add salt and mix the water, the sodium and chlorine atoms split apart (we call these “ions”) and “become one” with the water.
Again, everything is one phase because any region of the salt water has the same properties as any other region. We call this a solution.
A solution occurs when one substance dissolves another substance, the way water dissolves salt. In a solution, one phase absorbs another. You have one of the same phases which is now chemically different, but physically almost the same.
Water and salt yields salt water. Salt water and more water yields less-salty water. The dissolved salt would spread throughout all of the water, so as long as some atoms of water touch other atoms of water, the result is still a single phase.
But if you add oil to water, it is easy to distinguish from water. Thus, water is one phase and oil is another phase.
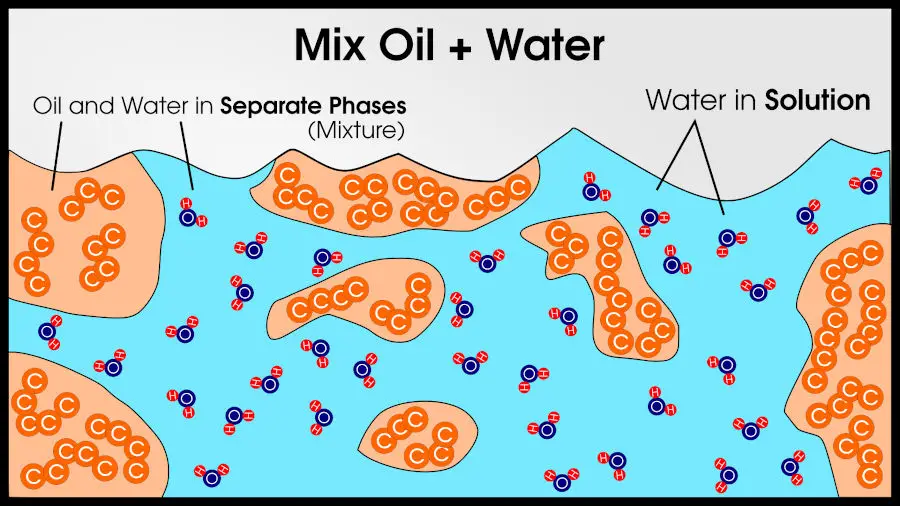
Different phases can also be chemically identical. Water and ice are chemically identical (they are made of the same atoms, 2 hydrogen per 1 oxygen), but since it’s still easy to tell the difference between water and ice, they are different phases.
Compounds and solutions (and elements) are made of only a single phase. Mixtures have multiple phases.
For example, you could make a mixture of 3 compounds: salt, water, and oil. You will end up with two phases: pure oil and a solution of salt water. Since there are two phases, the final result is a mixture, according to basic chemistry definitions.
In a compound, combining 2 or more phases yields a completely different phase.
In a solution, combining 2 or more phases yields one of the original phases.
In a mixture, combining 2 or more phases does nothing. Each phase stays distinct.
If you mix sand and water, it is easy to distinguish which part is sand and which part is water. There is no “sandy water,” just a mixture of sand and water.
Alloys are Solid-Solutions
Alloys are solid-solutions. That’s just like the regular solutions you usually think about, but solid. Freeze that salt water and you’ll have something similar to an alloy!
I think it’s important to mention that alloys are not defined as a solid solution of metals. That’s just a property they happen to have (in most cases).
“Alloy” specifically refers to the fact that the metal is not a pure element. If there are chemical bonds, the material wouldn’t act like a metal, so it wouldn’t be an alloy. Intermetallics are a special case that falls in the gray area; you can read more about them below.
For more on alloys, read this dedicated article!
Composites are Mixtures
Composites are a combination of two or more materials. Since the materials remain distinct, the composite is a mixture (to use chemistry nomenclature).
Usually, composites include a matrix phase and a strengthening phase. The matrix’s job is to hold the strengthening phase together. One classic example of a composite is carbon fiber reinforced-polymers (CFRP). CFRPs have very strong carbon fiber, bound with a polymer matrix. The fibers make the material very strong (in 1 direction) and the matrix holds the fibers in place.
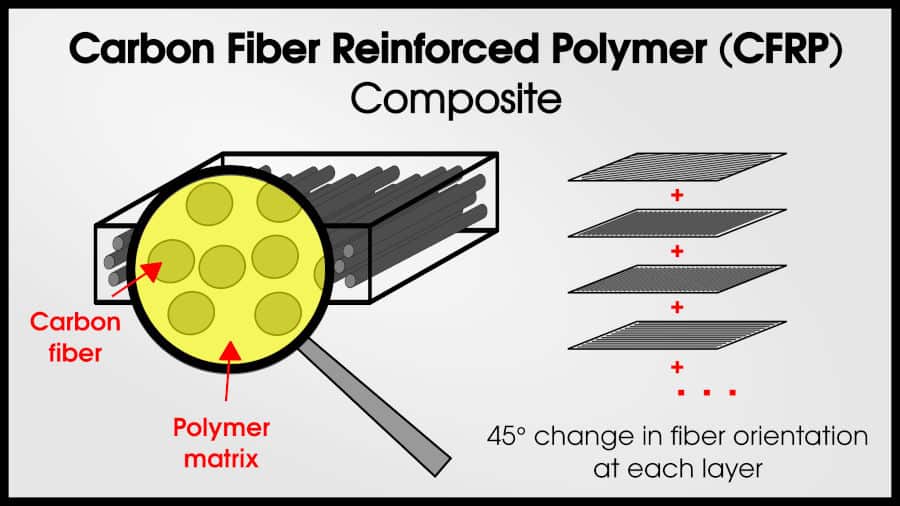
Importantly, the phases (fiber and matrix) remain distinct.
Alloys vs. Metal-Metal Composites
By now, I hope the difference between a metal alloy and a metal-metal composite is obvious.
Let’s review.
The metal alloy is a single phase (exceptions later) solid solution. The metal-metal composite has two metals with different functions and macro-separation.
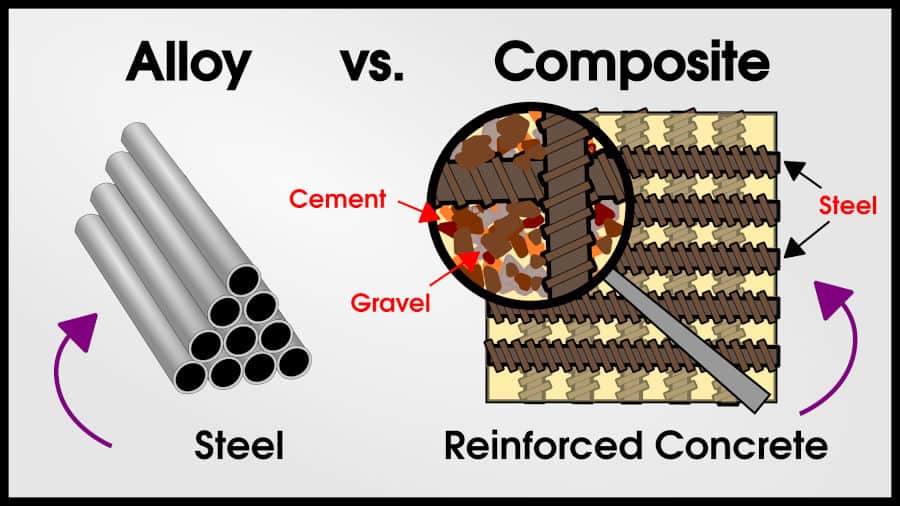
Why might one be better than the other?
When you create an alloy, you are essentially modifying the properties of one of the phases. For example, suppose you made an alloy of nickel and copper. You probably want a material that is close to nickel (or copper) but with some slight improvements.
If you add 5% copper to nickel, the nickel alloy will increase in strength (even though copper is weaker than nickel). This is because of solid-solution strengthening, which is a topic for another article.
If you glued a block of pure copper to a block of pure nickel (95% nickel by volume), the strength would be 95% strength of nickel +5% strength of copper.
Let’s look at this in a graph
So, does that mean that alloys are simply better than metal-metal composites? No.
Strength is one property that increases with alloying elements. Other properties decrease, or stay the same.
Conductivity, for instance, decreases when you add alloying elements. Alloying nickel with 5% copper will result in a less conductive alloy (even though copper is more conductive than nickel).
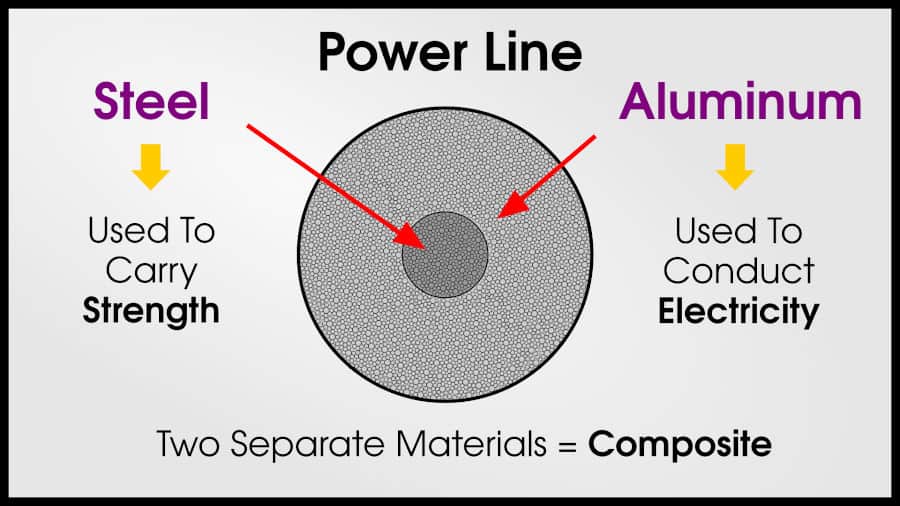
Power lines often use a composite of steel and aluminum. The aluminum conducts electricity (less alloying = better conductivity), while the steel provides strength (more alloying = better strength). The aluminum and steel alloys are separate because engineers want to preserve the conductivity of the aluminum.
In alloys (solid-solutions), the individual atoms still interact with each other. When we have an alloy of 50% copper and 50% nickel. Every copper atom is affected by nearby nickel atoms, and every nickel atom is affected by nearby copper atoms. The effect isn’t huge because there is no chemical bonding, but it still makes a difference.
In a composite with a block of copper glued to a block of nickel, only a tiny fraction of atoms at the interface interact with each other (actually if there’s glue, no nickel atoms interact with copper atoms!).
This is why metal alloys and metal-metal composites can have different properties, even though they have the same overall chemical composition.
The Gray Area: Multi-Phase Alloys and Intermetallics
It’s important to remember that terms like “alloy,” “composite,” “compound,” “ceramic,” “metal,” and “polymer” are just words. Most materials do fit neatly into these boxes, but some don’t.
Please, don’t get stuck in a semantic argument. It may be fun to argue whether “air” is an engineering material (I think yes because it has direct influence on properties like conductivity in foams or double-pane windows), but don’t worry about semantics.
That said, I do want to present my thoughts on two materials in the “gray area:” multi-phase alloys and intermetallics.
In truth, tons of alloys are multi-phase. Usually, the best structural materials are multi-phase. Steel and superalloys both have two (or more!) phases.
Because these alloys have more than one phase, they are technically mixtures. Well, they’re mixtures of solid solutions, similar to the salt water and oil example I provided at the beginning of this article.
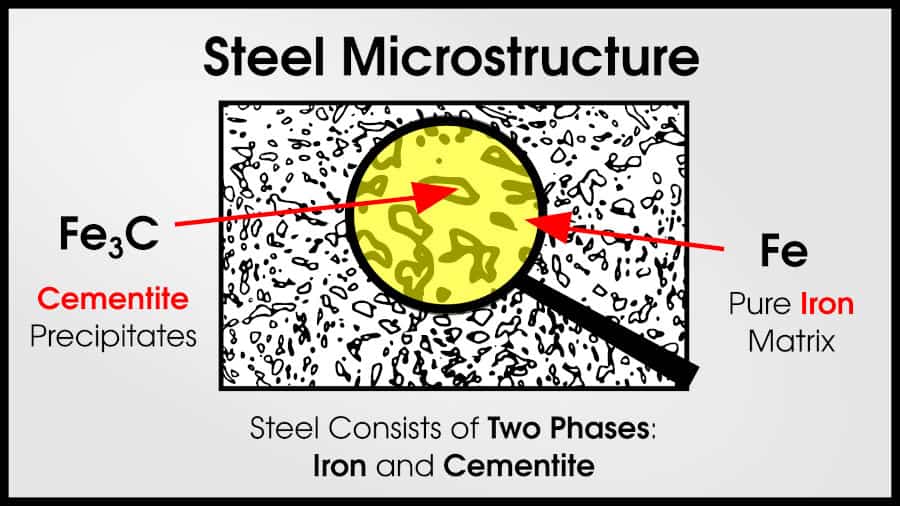
Steel microstructure often has an iron matrix with cementite (Fe3C) particles that make the steel strong.
Superalloys often have a Ni matrix with intermetallic precipitates that make the superalloy strong. (Superalloys may sometimes have 10 or more different phases!)
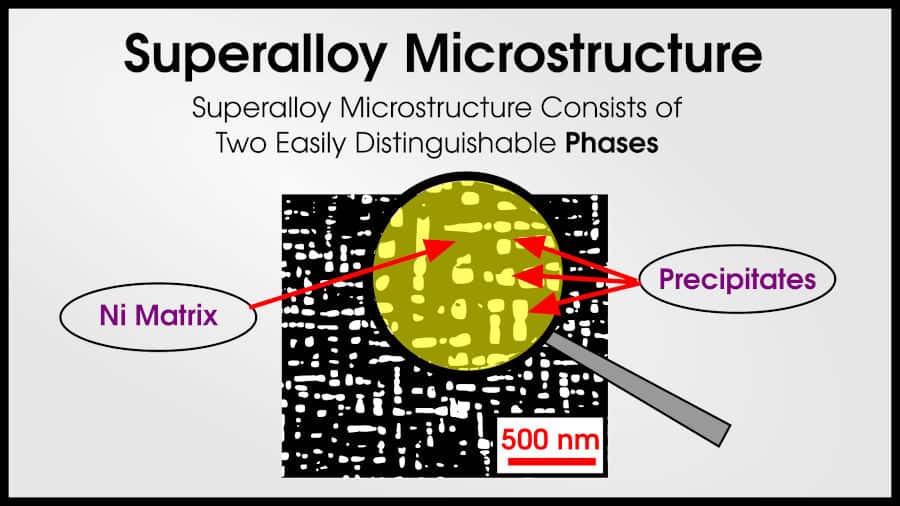
If you thought that this resembles a smaller version of the CFRP composite, you are absolutely right! A ductile matrix and strong reinforcing phase is a fantastic combination to make structural materials.
The only real difference is length scales. In these multiphase alloys, the size of each phase is very small. The phases are not glued together, but arise from thermodynamic precipitation. Furthermore, the alloys act like metals.
For these reasons, multiphase alloys are generally classified as alloys instead of composites. But if I was your professor and you want to argue that superalloys should actually be super-composites, I’ll listen!
The second hard-to-categorize material I want to discuss is intermetallics. Intermetallics are ordered metal “alloys.” That means that they have a stoichiometric ratio of metal elements. Each metal atom needs to sit at a specific site in the crystal lattice.
For example, perhaps the most-used structural intermetallic is γ’ (pronounced “gamma prime”), which is the strengthening phase in superalloys. γ’ looks like a regular face-centered cubic crystal, except that one atom always sits on the faces, and the other atom always sits on the corners. In a solid-solution face-centered cubic crystal, it would not matter which atoms were on which site.
In this way, intermetallics are very similar to compounds. In fact, I prefer the term “intermetallic compound” to “intermetallic alloy.” It’s hard to argue that intermetallics have chemical (rather than metallic) bonds, however, so they are rarely considered compounds outright.
If I had to choose how to classify intermetallics, I would say that it depends on the intermetallic in question. Generally, intermetallics behave like ceramics (Fe3C is definitely a ceramic, but some people call it an intermetallic because it appears so often in metal systems. Most intermetallics have properties similar to Fe3C), so I would say most intermetallics should be classified as ceramic compounds.
However, there are some intermetallics that behave like metals. I would consider these to be alloys, rather than ceramics. In particular, Ni3Al (the γ’ I was just talking about) and NiTi (shape memory alloy) are definitely “alloys” rather than ceramics.
Nomenclature Practice
I hope by now, you can easily tell the difference between alloys, composites, and compounds. But if you want to practice your nomenclature, here are some tricky questions! Some people will disagree with how I’ve categorized materials, and that’s okay. Feel free to comment your own answers if you want!
What is Glass?
Glass is an amorphous ceramic compound. It’s a ceramic, with chemical bonds, but the atoms are not arranged in a nice crystal lattice like all the other examples on this page.
What is PVC?
PVC is a polymeric compound.
What is Wood?
Wood is a polymeric composite (cellulose fibers in lignin matrix).
What is Iron?
Iron is a pure metal (not an alloy).
What is Steel?
Steel is an alloy (of iron and carbon).
What are Superalloys?
I think this makes sense as an alloy, although I would understand the argument that superalloys are a composite between an FCC alloy and L12 intermetallic. Since it behaves like a metal, almost everyone considers it an alloy
What are Power Lines? (Aluminum and Steel)
Power Lines are metal-metal composites! The aluminum conducts electricity, and the steel provides structural support.
What is Foam?
Foam is a solid-gas composite. The solid portion can be a polymer (like styrofoam that you are used to), or even a metal or ceramic!
Final Thoughts
Remember, nomenclature is a tool to help scientists and engineers make quick assumptions. Not everything falls into neat boxes, and quibbling about a definition won’t get you anywhere.
I once read an argument that claimed that almost every material was a composite if you looked at micro-scale segregations within the material. That is true, but if everything is a composite, the word is no longer useful.
In common usage, “composite” means that different materials are intentionally joined to achieve a new combination of properties.
“Alloys” are metals. Many alloys are a single phase, like salt water, but some alloys have multiple phases, separated by very tiny amounts, that overall still behaves like a metal.
Further Reading
If you want to learn more about alloys, here is an entire article just about alloys.