
When I was a kid, I thought all metals were magnetic, and nonmetals were not magnetic. Then I learned about ferromagnetism, diamagnetism, and paramagnetism and I thought that Magneto (from X-men) could only control iron. It wasn’t until I took advanced university physics classes that I actually learned the relatively simple concepts about magnetic materials.
The following metal elements can be magnetic:
- Iron (Fe) [1]
- Nickel (Ni) [2]
- Cobalt (Co) [3]
- Gadolinium (Gd), only below room temperature [4]
- Dysprosium (Dy), only at very cold temperatures [5]
- Erbium (Er), only at very cold temperatures [5]
- Holmium (Ho), only at very cold temperatures [6]
There are also many compounds that can be magnetic, including:
- Hard Ferrites, which are ceramic, hard ferrimagnets with a hexagonal crystal structure. Examples include BaFe12O19 and SrFe12O19 [7]
- Soft Ferrites, which are ceramic, soft ferrimagnets with a hexagonal crystal structure, and the formula MO.Fe2O3, where M represents transition metals like nickel, manganese, or zinc [8]
- SmCo-type hard ferromagnets, which include SmCo5 or compounds of the general formula Sm2(Co,Fe,Cu,Zr)17, such as Sm2Co17 [7]
- NdFeB alloys, which are magnetic because of the hard ferromagnetic phase: Nd2Fe14B [7]
Of course, that’s the question I think you’re asking. In reality, all materials are magnetic–they just don’t stick to magnets. I gave you a list of ferromagnetic elements (and ferrimagnetic compounds) which do stick to magnets, but in certain cases, even metals that do not normally stick to magnets can be affected by a magnetic field.
Oh, and if you tried to see if a nickel coin was magnetic, don’t bother. I’ll talk about this later, but U.S. nickels are only 25% nickel, which is enough to make it completely non-ferromagnetic.
Outline
- What Kinds of Magnetism Exist in Elements?
- Are There Ferromagnetic Materials Besides Iron?
- What is Ferromagnetism?
- Advanced Concepts about Permanent Magnets
- List of Ferromagnetic Materials
- List of Ferrimagnetic Materials
- Can Magnetism Appear in non-magnetic materials?
- Hard and Soft Magnets
- Final Thoughts
- References and Further Reading
What Kinds of Magnetism Exist in Elements?
There are 3 kinds of magnetism in elements: paramagnetism, diamagnetism, and ferromagnetism.
Small disclaimer
Okay, fine there’s also antiferromagnetism, but only chromium has this type of magnetism at room temperature, and antiferromagnetism behaves almost exactly like paramagnetism anyway.
If you want to learn more about paramagnetism, diamagnetism, and other forms of magnetism that don’t occur in pure elements, I wrote a comprehensive article about magnetism in materials.
Paramagnetic and diamagnetic materials don’t stick to magnets, so most people would just consider them “non-magnetic.” These materials do respond to magnetic fields, but the effect is so weak that you wouldn’t be able to tell without specialized laboratory equipment.
The effect of a material’s internal magnetic field aligning with an external magnetic field is called magnetic polarization, and all materials polarize to some extent. Ferromagnetic (and ferrimagnetic) materials can spontaneously polarize, which means that the internal magnetic field can be aligned even without an external magnetic field.
“Spontaneous magnetic polarization” is the technical term for what most people mean when they say “magnetic.”
The rest of this article will focus on ferromagnetism (and ferrimagnetism), but just in case you have a homework question that needs you to list types of ferromagnetic, diamagnetic, and paramagnetic materials, here is a periodic table that shows what type of magnetism every element has.
(By the way, this table only applies to elements at room temperature)
Are There Ferromagnetic Materials Besides Iron?
Yep. Most metals are not ferromagnetic, but the list is longer than just iron. The term involves the latin root for iron, “ferro,” because iron is the most widely-used element which has ferromagnetism.
Even though ferromagnetism is also found in nickel and cobalt, if you find a random piece of metal that sticks to a magnet, the metal is probably an iron alloy (steel).
It’s really rare to see nickel- or cobalt-based alloys in everyday life, since steel is cheaper and stronger for most applications.
Even “nickels,” the U.S. 5-cent coin, is only 25 wt% nickel (the rest is copper). Copper is not ferromagnetic, and with so much copper, the cupronickel alloy is overall not ferromagnetic.
It’s also possible to have nonmagnetic steel alloys. Some stainless steels have a different crystal structure than plain iron, and these “austenitic” stainless steels are not ferromagnetic. So, if you see a silvery metal that’s more dense than aluminum, even if it’s not magnetic, that’s probably still steel (most metals you encounter in daily life will be aluminum or steel).
Now you know which metal elements are ferromagnetic (aka “layperson’s magnetic”), but you’re still reading, so I’ll assume you want to know what actually happens during ferromagnetism, and why ferromagnets are thousands or millions of times stronger than paramagnets or diamagnets.
What is Ferromagnetism?
Ferromagnetic materials are divided into magnetic domains, or Weiss domains. These are hard to conceptualize because they don’t really have anything to do with crystal structure. There are many domains per crystal grain.
Each domain is a region of material with a uniform magnetization. This means that all the atoms in a domain contribute their atomic magnetic moment in the same direction.
Initially, domains usually don’t align in the same direction, which is why a piece of iron doesn’t usually produce its own magnetic field.
Once an external magnetic field is applied, however, the domains are affected by the field so they align parallel to the external magnetic field. This produces an internal magnetic field from within the ferromagnet.
If you pick up a paperclip with a magnet, you can create a chain of paperclips. When the domains in the steel of one paperclip align, the paperclip becomes magnetic and can produce a weaker magnetic field in another paper clip.
The magnetic moments of these domains are strong enough that–even if you remove the external magnetic field, the internal magnetic field keeps the domains aligned, so they keep producing an internal magnetic field. That’s why you can magnetize iron, even once the magnet is no longer touching the iron.
Magnetic Susceptibility is the way that the domains line up. Since domains in ferromagnetic materials strongly align with the external magnetic field, ferromagnets have large, positive susceptibilities.
A magnetic field flowing through a ferromagnetic material might be as much as 10,000 times stronger than the same magnetic field flowing through a vacuum.
Click to expand for a brief explanation of paramagnetic and diamagnetic materials
In paramagnetic materials, the domains still align with the external field. However, since they are weak (too weak for you to notice without special equipment), they can’t support their own internal magnetic field. Once the external field is removed, the domains go back to a random orientation.
In other words, paramagnetic materials have weak, positive susceptibilities. To give you an idea of how weak this is, a magnetic field flowing through a paramagnetic material might be 0.001% stronger than the same magnetic field flowing through a vacuum.
In diamagnetic materials, the domains actually align against the external field. However, this is even weaker than paramagnetism, and the domains return to random once the external field is removed. The only time you’d see a material repel a magnet is in the Meissner effect with superconductors.
Otherwise, diamagnetic materials have weak, negative susceptibilties. A magnetic field flowing through a paramagnetic material might be 0.00001% weaker than the same magnetic field flowing through a vacuum.
Advanced Concepts about Permanent Magnets
Here are a couple advanced ideas in ferromagnetism. I won’t go into too much detail so the paragraphs are collapsed by default, but if you are interested in magnetism you can learn a few vocabulary words to search when you’re done with this article.
Hysteresis
Because ferromagnets can stay magnetized even after the external magnetic field is removed, if you applied a positive, then negative magnetic field, you would see a property called hysteresis. Since I already explained this in a previous article, I won’t repeat myself here.
Depending on the shape of the hysteresis curve, materials might be considered “hard’ or “soft.” This “magnetic hardness” separates magnetic materials by application. I’ve included a list of hard and soft magnets later in this article.
If you want to learn more about hysteresis, check out this article I wrote about magnetic hysteresis.
Curie Temperature
At a certain temperature all ferromagnets and ferrimagnets become paramagnetic. This temperature is called the Curie temperature (named after Pierre Curie, not Marie Curie).
Do you know how solids are held together by bonds, but at a certain temperature the atoms are vibrating so rapidly that the bonds loosen and break, so the solid becomes a liquid or gas? This is the same principle behind the Curie temperature.
| Ferromagnetic Element | Curie Temperature (°C) |
| Iron (Fe) | 770 |
| Nickel (Ni) | 354 |
| Cobalt (Co) | 1121 |
| Gadolinium (Gd) | 20 |
| Dysprosium (Dy) | -168 |
| Erbium (Er) | -253 |
| Holmium (Ho) antiferromagnetic between 20K and 133K, and paramagnetic above 133K | -253 |
At the Curie temperature, the magnetic domains have so much thermal energy that they can constantly re-align, preventing them from keeping their magnetic alignment after the external magnetic field is removed.
At the Curie temperature, ferromagnetism is lost and the material simply becomes paramagnetic.
The Origin of Magnetism
Ferromagnetism’s origin has to do with unpaired electrons and quantum mechanical effects. I didn’t do great in my PhD quantum mechanics class, so I’ll link a hypertext that does a better job explaining it than I can [9].
Ferrimagnetism
There is another class of magnet, called ferrimagnets, which behave almost exactly the same as ferromagnets. Ferrimagnets are ceramic compounds so their magnetic origin is slightly different than ferromagnets, but in most cases something that applies to ferromagnets will also apply to ferrimagnets. If you would like to learn more about this distinction, and types of magnetism in general, check out this article.
Most magnets you see in daily life are ferrimagnets.
Ferrimagnets work because of partial antiparallel alignment, which means the atomic magnetic moments align opposite each other, but since there is an uneven number of these moments, they can’t fully cancel.
For example, the most-known ferromagnetic material is magnetite (Fe3O4), the main component of lodestones [10].
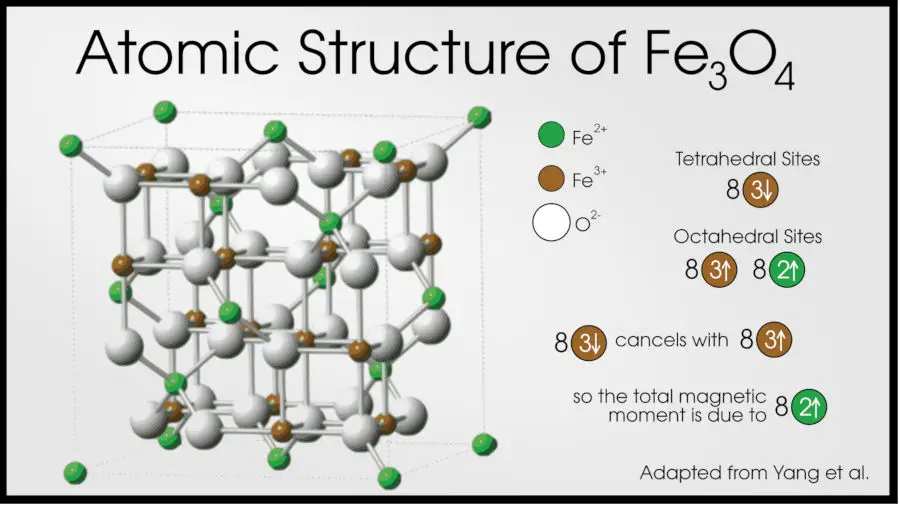
In magnetite, each iron atom in a unit cell contributes to the magnetic moment, but one has a different valency than the others (two Fe3+ and one Fe2+). The Fe3+ atoms align to oppose each other, canceling each other out, but the Fe2+ is free to align with an external magnetic field.
Ferrimagnets are ceramics, so they usually don’t conduct electricity. This is good for making permanent magnets, because it means that the material won’t have eddy currents and nearby electric fields are unlikely to remove the internally polarized magnetic field.
If you would like to learn more about the distinction between ferromagnetism and ferrimagnetism, or other types of magnetism, check out this article.
List of Ferromagnetic Materials
I already gave you this list, but let’s explore the list of ferromagnetic materials a bit more. We can also divide ferromagnets into “hard’ and “soft” magnets based on their hysteresis curve.
Common Ferromagnetic Elements
- Iron (Fe)
- Cobalt (Co)
- Nickel (Ni)
The most common ferromagnetic element is iron. Most iron alloys (or steels) are also ferromagnetic, although some steel alloys–called “austenitic stainless steel” are not ferromagnetic.
Nickel and nickel alloys are also ferromagnetic, up to a point. For example, alloying nickel with copper will reduce the Curie temperature–at around 65 wt% nickel, the Curie temperature is at 0°C, so it would be magnetic in your freezer, but not at room temperature. U.S. Nickel coins are only 25 wt% nickel, so you can imagine that their Curie temperature drops below absolute zero, until it isn’t ferromagnetic at all [11-14].
The final “common” ferromagnetic element is cobalt. In daily life you likely use cobalt compounds or alloys containing small amounts of cobalt, but you are unlikely to use a cobalt-base alloy. That’s why people rarely remember that cobalt is actually ferromagnetic.
Rare Earth Ferromagnetic Elements
The following rare earth elements are also ferromagnetic.
- Gadolinium (Gd)
- Dysprosium (Dy)
- Erbium (Er)
- Holmium (Ho)
Ferromagnetic Alloys
Examples of Soft Ferromagnetic Alloys [8]
- Iron-Silicon alloy
- Nanocrystalline alloys like FINEMET or NANOMET
- Nickel-iron alloys (check below: permalloy, Mu-metal, Supermalloy)
- Permalloy (Ni-Fe alloy) Typical composition: ≈ 80 wt% Ni, ≈ 20 wt% Fe
- Mu-metal (Ni-Fe alloy)
Typical composition: ≈ 77 wt% Ni, ≈ 16 wt% Fe, ≈ 5 wt% Cu, ≈2 wt% Cr or Mo - Supermalloy (extremely high magnetic permeability)
Composition: 75 wt% Ni, 20 wt% Fe, 5 wt% Mo
Examples of Hard Magnetic Metals/Alloys/Materials [7]
- Alnico
Typical composition: 35 wt% Fe, 35 wt% Co, 15 wt% Ni, 7 wt% Al, 4 wt% Cu, 4 wt% Ti - SmCo-type magnets
Based on the SmCo5 ferromagnetic phase SmCo5, or Sm2(Co,Fe,Cu,Zr)17. - Neodymium magnets
Have the ferrimagnetic [15] phase Nd2Fe14B, which may be processed via sintering or melt spinning. I believe these are the strongest commercially available magnets.
Ferromagnetic Compounds
It’s also possible to have ferromagnetic compounds. When atoms take certain positions in a crystal structure, the electron interactions change. The Huesler structure is an intermetallic compound that usually has interesting magnetic properties.
A full Huesler has structure X2YZ where X and Y are transition metals and Z is in the p-block.
Most Huesler’s of the form X2MnZ have ferromagnetic properties, such as [16]
- Cu2MnAl
- Ni2MnAl
- Ni2MnGa
- Ni2MnSn
- Ni2MnSb
- Au2MnAl
- Pd2MnSn
- Pd2MnZ
For these examples, having the Mn atoms spaced apart from each other is enough that Mn can become ferromagnetic, giving the whole crystal ferromagnetism.
There are also Co-based Heuslers, such as [17]
- Co2MnAl
- Co2MnSi
- Co2MnGa
- Co2FeAl
- Co2FeSi
- Co2FeGa
- Co2TiAl
- Co2TiSi
- Co2TiGa
- Co2NbAl
- Co2NbSi
- Co2NbGa
- Co2ZrAl
- Co2ZrSi
- Co2ZrGa
- Co2HfAl
- Co2HfSi
- Co2HfGa
SmCo5, and Sm2(Co,Fe,Cu,Zr)17 are also ferromagnetic intermetallic compounds, but Nd2Fe14B is a ferrimagnetic intermetallic compound [15].
List of Ferrimagnetic Materials
Some common structures that allow ferrimagnetism are the garnet structure such as yttrium iron garnet (Y3Fe5O12); cubic ferrites (MFe2O4) and hexagonal ferrites (MFe12O19) where M is a metal that has a valency of 2, such as Fe3O4, NiFe2O4, or PbFe12O19; and pyrite, which is an iron-sulfur alloy.
Ferrimagnetic Materials
- Garnets (M3Fe5O12 where M is usually a rare earth metal) [18] are usually hard magnets. Some examples of garnets are [19]:
- Y3Fe5O12
- Er3Fe4O12
- Sm3Fe5O12
- Gd3Fe5O12
- Cubic Ferrites
where M is a divalent metal) are usually soft ferrimagnets. Examples include [20]:
- Fe3O4
- NiFe2O4
- MnFe2O4
- MgFe2O4
- ZnFe2O4
- CdFe2O4
- BaFe2O4
- Hexagonal Ferrites (MFe12O19) are usually hard ferrimagnets. Hexagonal ferrites can be further classified as [21]:
- M-type ferrites, such as BaFe12O19, SrFe12O19, and cobalt–titanium substituted M ferrite, Sr- or BaFe12−2xCoxTixO19 (CoTiM).
- Z-type ferrites (Ba3Me2Fe24O41) such as Ba3Co2Fe24O41, or Co2Z.
- Y-type ferrites (Ba2Me2Fe12O22), such as Ba2Co2Fe12O22, or Co2Y.
- W-type ferrites (BaMe2Fe16O27), such as BaCo2Fe16O27, or Co2W.
- X-type ferrites (Ba2Me2Fe28O46), such as Ba2Co2Fe28O46, or Co2X.
- U-type ferrites (Ba4Me2Fe36O60), such as Ba4Co2Fe36O60, or Co2U
- Pyrrhotite, or pyrite
- Nonstoichiometric Fe_1-xS_x, where X is 0 to 0.2
Ferrimagnetism cannot be found in pure elements because it relies on magnetic asymmetry within a crystal structure of multiple elements.
Can Magnetism Appear in Non-magnetic Materials?
Yes! There are a few ways this can happen, but they happen because of basic physics principles, rather than some material property.
Electromagnets
Electromagnets can be created in any electrically conductive material. They are based on a principle called Lorentz force, which basically says that a moving electron generates a magnetic field.
By wrapping an electrically conductive wire around a cylinder, the electrons flowing through the wire produce a magnetic field, creating an electromagnet.
In principle, the “core” can be any material including air, but in most cases the core should be ferromagnetic. As we discussed, the ferromagnetic material will align with the magnetic field produced by the wire, and create stronger magnet than the wire alone.
However, if you wanted to create a magnet out of a nonmagnetic metal like copper or aluminum, you could form the conductive metal into a wire, shape the wire into a spiral, and run a lot of current through it.
Moving Non-Magnetic Materials with a Magnet: Lenz’s Law
You may have seen examples of powerful magnets being used to move quarters, copper blocks, or aluminum soda can tabs (this is one method of sorting recycled aluminum).
This phenomenon can happen in any electrically conductive material–it’s basically the follow-up to the Lorentz force. A moving current produces a magnetic field, and a moving magnetic field produces a current.
It turns out that a moving magnetic field can create a current–and the current creates another magnetic field in the opposite direction of the original one.
So, if you quickly move a powerful magnet near a dime (mostly copper with a bit of nickel), the dime will move even though it wouldn’t be attracted to a magnet standing still!
Similarly, if you dropped a magnet through an electrically conductive, non-ferromagnetic tube, the magnet would slow down and the induced magnetic field opposes the natural magnetic field of the magnet.
The only property that matters for this is conductivity. So as it turns out, dimes are more likely than nickels to be affected by a moving magnet, even though nickels have a higher percent of ferromagnetic Ni. Neither coin has enough Ni to be ferromagnetic, but Cu is more conductive than Ni, so dimes (with higher Cu content than nickels) will be more affected by Lenz’s law.
Metal Detectors
Metal detectors also work on the principle of Lenz’s law. It detects magnetic fields, but not from ferromagnetism–instead, the magnet in the metal detector induces an electric current in the detected metal, and that metal produces a magnetic field.
The detector senses this induced magnetic field, which means it is actually sensing electrical conductivity, not magnetism. It’s also important for the metal detector to be constantly moving. If you slowly placed a metal in front of a metal detector, so slowly that the induced magnetic was too weak, the detector would not be able to sense it.
Hard and Soft Magnets
Of course, magnetic “hardness” or “softness” depends on perspective, but For applications and further explanation of hard magnets, but sure to check out my article explaining the difference between hard and soft magnets.
| Examples of Soft Magnets | Examples of Hard Magnets |
| Ni-Fe alloys, Silicon Steel Soft Ferrites, Cubic Ferrites Some Nanocrystalline alloys | Alnico, NdFeB Magnets Samarium Cobalt, Hard Ferrites Garnets, Hexagonal Ferrites |
Final Thoughts
Well, that was a lot! In this post, you learned that “magnetic” materials actually means “ferromagnetic” of “ferrimagnetic” materials.
I hope I have also provided a more extensive list about magnetic materials than you can find anywhere else on the internet! I’ve divided the list into ferromagnetic elements, hard ferromagnetic alloys, soft ferromagnetic alloys, and ferrimagnetic compounds.
You also learned about ways that “non-magnetic” materials can still be influenced by magnetic fields!
References and Further Reading
If you want to learn more about the origin of magnetism, and all magnetic types (like diamagnetism or antiferromagnetism, which don’t respond to ordinary magnets), check out this article.
If you want to understand more about ferro/ferrimagnetic properties (or want to learn about hysteresis in general), I recommend my article about magnetic hysteresis curves or my more-technical article about graphing magnetic properties.
If you want a quick summary of different types of magnetism, check out this article by the University of Birmingham.
And if you noticed in my periodic table that Chromium is “antiferromagnetic,” and want references, you can find them here and here.
[1] Lau, F. and Lei, H., 2005. “Curie Temperature Of Iron.” The Physics Factbook. (2005).
Curie point of iron is 1043 K
Curie point of nickel is 627 K
[3] Sinclair, Y. “Curie Temperature Of Cobalt.” The Physics Factbook. (2005).
Curie point of cobalt is 1121 ºC
Curie point of gadolinium is 292.5 ± 0.5 K
Curie point of dysprosium is 105 K, Curie point of erbium is 20.4 K
Curie temperature of holmium is 20 K, and Nėel temperature is 133 K
[7] “Magnetic Materials: Hard Magnets.” University of Birmingham.
Great explanation and list of hard magnets
[8] “Magnetic Materials: Soft Magnets.” University of Birmingham.
Great explanation and list of soft magnets
Excellent hyperscript textbook by Prof. Dr. Helmut Föll; I’ve directly linked to his explanation of ferromagnetism.
Image of Fe3O4 adapted from this reference.
[14] Williams, E. H. “Magnetic Properties of Copper-Nickel Alloys.” Physical Review 38.4 (1931): 828.
Reference that Nd2Fe14B is ferrimagnetic, not ferromagnetic
Examples of Huesler ferromagnets
More examples of Huesler ferromagnets
[18] Mason, T., 2016. “Magnetic Ceramics.” Encyclopedia Britannica.
General formula for garnet ferrites
Examples of common ferrimagnetic garnet ferrites
[20] Mahan, Gerald D. “Crystals.” Encyclopedia Britannica.
Examples of cubic ferrites
Examples of hexagonal ferrites
Here is a quick summary on types of magnetism.
Here is a scientific paper deriving the antiferromagnetic behavior of chromium: Entel, P. “Magnetic susceptibility of antiferromagnetic chromium.” Journal of Magnetism and Magnetic Materials 6 (1977): 134-138.
Click here to read a scientific paper measuring the magnetic susceptibility of chromium over a wide temperature range: McGuire, T. R., and C. J. Kriessman. “The magnetic susceptibility of chromium.” Physical Review 85.3 (1952): 452.