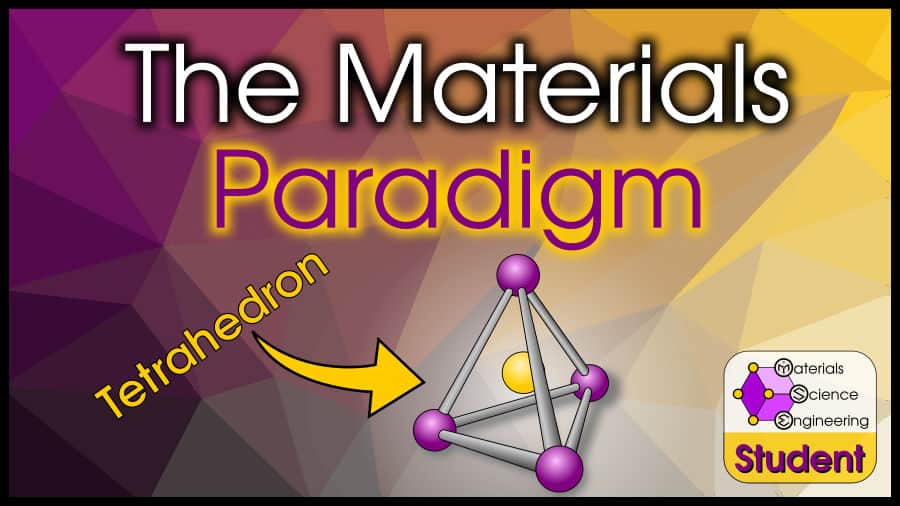
Why is the Materials Science Tetrahedron so Important? If you don’t understand the significance of the materials science and engineering tetrahedron (also called the materials paradigm), don’t worry. For most of my undergraduate studies, I was annoyed and confused by how often professors would mention the materials tetrahedron–I didn’t see what it added to the lecture. To a seasoned student in materials science, processing, structure, properties, and performance are distinct, easily-identifiable concepts. What was the significance of the materials paradigm?
The materials science tetrahedron defines the scope of materials science and engineering. The tetrahedron emphasizes the four interdependent, distinct aspects of materials science: processing, structure, properties, and performance. These “corners” of the tetrahedron may seem simple, but from an educational point of view, it is critical to identify what students learn about each corner.
Materials science is a highly interdisciplinary field, so we need guidelines to decide what “belongs” to materials science. These distinctions aren’t so important after graduation, but as an educational subject, materials science needs to meet certain criteria.
Since materials science is so broad, there is a dangerous possibility: what happens if students from different programs learn vastly different subjects? If that happened, companies would no longer know what a degree in materials science actually represents. Enter accreditation.
In particular, there is something called ABET (Accreditation Board for Engineering and Technology). This board certifies most STEM departments at American universities. If the department is ABET-accredited, companies know that every student who graduates has met a certain standard and has certain knowledge.
Employers can trust that–even if you graduated from a small, unrecognizable school–as long as the department is ABET accredited, you have the same fundamental knowledge as the best students from the best universities.
There are many guidelines that university programs need to follow to maintain their accreditation, but one of these is the educational guidelines. For each discipline, ABET specifies learning outcomes that every student needs to meet.
For materials science, these learning outcomes are often centered around the materials tetrahedron. It is a paradigm, or philosophy, of materials science. If the breadth of materials science could be illustrated in a single diagram, that diagram would be the materials tetrahedron. If professors constantly ask you to explain how your project relates to each corner of the materials tetrahedron, they are just making sure you think like a materials scientist.
So if you were wondering why so many people keep mentioning the materials science tetrahedron–it is pedagogical. It represents the philosophy of materials science and engineering
Of course, as a pedagogical tool, it also contains a very useful framework. Explaining the materials tetrahedron will provide an overview of everything materials science. Let’s discuss a few ways to visualize the tetrahedron, and then look at each point in turn.
Outline
Visualizing the Materials Tetrahedron
The most popular visualization of the materials tetrahedron is given at the top of this page (probably because someone drew a beautiful version and put it in the public domain). Each part of the tetrahedron (processing, structure, properties, performance) has its own point and relates to each other point. The “official” version of the tetrahedron that I use on this site also has “characterization” in the center of the tetrahedron.
The most basic illustration of the tetrahedron is drawn like so:
Different people will draw different points on “top” (or arranged so that there is no top). I like to put performance on top, because I view the other 3 points as leading to performance.
Sometimes, people will simply draw a triangle.
The triangle combines properties and performance into a single point. This model works really well from a research and development perspective.
When developing new materials, scientists often consider the processing-structure relationship, and then consider how that relates to the properties. If the scientist is not considering the final application of the material, but just wants to advance some aspect, there is no divide between ideal properties and ultimate performance.
On the other hand, for engineers who are performing analysis for materials selection, the distinction between properties and performance is critical. This engineer often needs to consider trade-offs between different properties to decide what material will have the best performance.
If you want to emphasize this thought process, you may like the collapsed materials tetrahedron–or the materials chain.
This diagram considers each of the four points in natural sequence. Processing affects structure, structure affects properties, and properties affect performance. From the other end, if you need a material that has a certain performance, you need to create a set of ideal properties. To get those properties, you need to create certain structures. The structures are created by processing.
Since I am often asked about the difference between materials scientists and materials engineers, I also made this slight variation on the materials tetrahedron.
This version emphasizes the (largely imagined) difference between materials scientists and materials engineers. There are so many exceptions to the rule that I’m not sure I should mention it, but there is a tendency for scientists to focus on the structure-property relationship, and engineers to focus on the processing-performance relationship.
For the sake of completeness, I will also mention Professor Donahue’s reimagined “materials pyramid.” In 2019, he presented an argument for adding a “sustainability/criticality” measurement.
This base of the pyramid represents a consideration for the ecological impact of the material. Personally, I think this “sustainability/criticality” metric can easily be considered a material property (one which is affected by processing, not structure), and so there is no need to modify the traditional materials pyramid.
But who knows? Perhaps we’ll see a gradual shift in the way the materials tetrahedron is portrayed. Do you think a change is warranted? Which version of the tetrahedron do you like the best?
In the meantime, you clicked on an entire article about the materials tetrahedron, so I’m prepared to deliver. Let’s talk about each point on the materials tetrahedron in excruciating detail.
Processing
Processing is a term that encompasses every way in which the material is changed. This can include recycling, mining raw ore, purifying the material, shaping the material, and more.
Typically, we consider two types of processing: primary processing and secondary processing.
Primary processing includes all of the processing steps to create usable material. This means digging the raw materials from the ground, chemically separating the useful bits, purifying the useful bits, and somehow creating a mass of material that someone else will buy.
Secondary processing includes all of the processing steps after you have the “final” material. This means forging, rolling, milling, cutting, extruding, polishing, heating, quenching, and possibly even doping.
For example, let’s look at steel. Steel starts as iron ore and carbon dioxide. The ore needs to be mined, separated from not-iron in the ore, alloyed, combined with carbon, and purified. Everything up until now was primary processing. Only now would you call the material “steel.”
Since you have the final product, the next steps are secondary processing. The steel may be cast into large ingots to sell to people. A factory may buy some of the steel, shape it, heat treat it, or coat it.
There is a small gray area where secondary processing changes the chemical structure. Continuing with the steel example, the company might add additional elements to the surface (as in case-hardening or doping). Depending on the degree that the material is redefined by the change, it’s possible that this change would still be primary processing (so everything that happened earlier–since it was necessary for this change, is also primary processing).
If you need points on a trick question, there’s the answer. To be honest though, there is little point in arguing about the difference in primary processing and secondary processing. Generally these are just terms of convenience so we can quickly talk about whether the material is still being created, or if the created material is being prepared for some other difference. Some textbooks may even define additional processing phases, such as “tertiary,” “quaternary,” or “advanced” processing.
Processing refers to all steps in which the material is changed to become more useful.
Processing is the way we affect the material’s structure.
Structure
Many (most?) materials science programs place the most emphasis on teaching the “structure” aspect of materials science.
Structure refers to the way a material is arranged at different length scales. This can be differences in atomic bonding, grains, precipitate arrangement, or even macro-scale architecture (although other engineers typically work at those higher length scales).
The most often-used example of structural difference is diamond vs graphite. Both diamond and graphite have the same chemical makeup (carbon), but vastly different atomic structures.
In graphite, the carbon is bonded into 2-dimensional sheets.
Diamond has a special crystalline arrangement of atoms called “diamond cubic.”
The difference in their structure leads to different properties (spoiler for the next section).
Graphite’s 2D layered structure makes it quite soft because the layers can slide past each other. That makes it excellent for lubrication.
Diamond’s crystal structure is especially good for strength. That is part of why diamond is the hardest naturally-occurring material.
We can also consider structure at a higher level. If graphite is made of many 2D sheets, what happens if we stack those sheets so they are aligned? They will have different properties than if they were randomized!
If the sheets are aligned, graphite will have anisotropic properties. That means they are not the same in every direction. For example, arranging graphite like this could allow good conductivity in the plane, but poor conductivity out of the plane. I’ll trust your imagination to see why this is useful!
So, what are some common structures?
At the atomic level, structure comes down to bonding. Are the bonds metallic, covalent, or ionic? Are atoms merely held together by van der Walls forces? How closely to atoms get to each other? What is the size difference between anions and cations? Are atoms arranged in a cubic, hexagonal, or some other crystal pattern?
When we look at groups of atoms, we can consider defects. We cover defects later in this series on materials science fundamentals, but defects are places where atoms don’t belong. For example, the material may have vacancies, or it may have extra interstitial atoms. (I know I’m not supposed to talk about properties yet, but adding extra atoms with different valence electrons is called doping and it’s extremely important to making semiconductors).
We can also consider phases–or different groups of atoms with the same ratios of elements. Usually, a material will have a main (ductile) phase–called the matrix–mixed with small, harder phases called precipitates. A common example of this occurs in many steels. (WARNING: I’m about to make an extreme oversimplification which is not technically true).
If you have a mixture of 90% iron and 10% carbon, you will actually get phases of Fe and Fe3C (in other words, 100% iron phase and 75% iron phase). The final result would be 60% Fe matrix and 40% Fe3C precipitates.(Again, HUGE oversimplification).
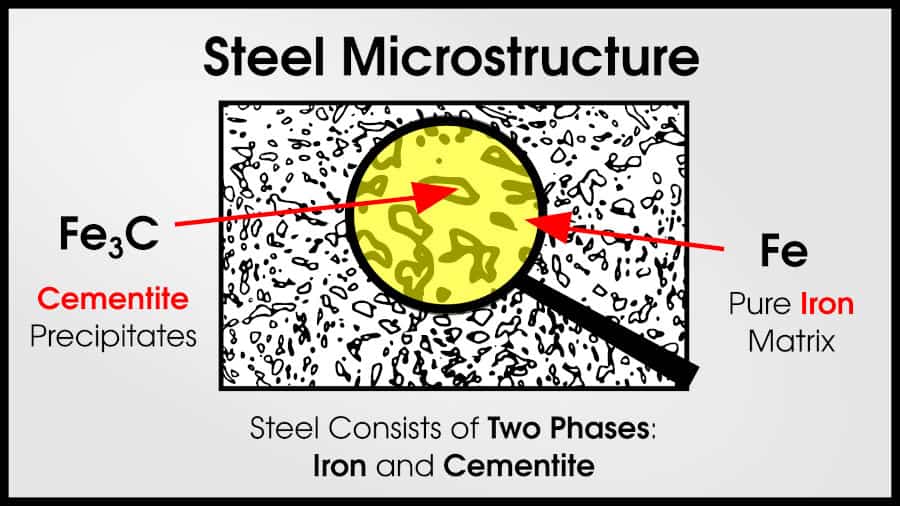
As we increase the length scale (we call this microstructure), it’s important to consider grains. Grains are groups of atoms that all have the same crystallographic direction. Each grain is a single crystal. Gemstones are a single grain, while most materials–including virtually all metals–are polycrystalline. That means they are made of many different grains.
At the macroscopic scale, our material may have voids, or unintentional holes. This is almost always bad, and processing plays an important role in avoiding voids.
The material may also be composite–it could be a porous foam (with intentional holes designed to decrease weight), or a combination of different materials designed to balance each other out. For example, concrete has rocks and cement (cheap and great for compressive strength) mixed with steel rebar (more expensive, but adds needed tensile strength).
A materials scientist would consider structures in concrete at every level: the grains and precipitates in the steel, the size and bonding in the concrete, and the macro-scale interaction between steel and concrete that balances for the desired properties.
Structure refers to the way sub-components of a material are arranged, especially on different length scales.
A fundamental assumption in materials science is that all properties are derived from the material’s structure (we can stretch this to include chemistry and chemical bonding by referring to electronic structure as well).
Properties
Properties are anything measurable about a material. In materials science, we typically focus on intrinsic or intensive properties. These are properties that are fundamental to the material, regardless of how much material there is. In contrast, we can also talk about extrinsic or extensive properties, which depend on how much material you have.
Examples of intrinsic properties: color, density, conductivity, strength, melting point.
Examples of extrinsic properties: volume, mass, energy, entropy.
For the sake of accuracy, sometimes intrinsic properties can become extrinsic. For example, ice cubes of different sizes may appear to have different colors
For a more universal example, conductivity is related to the number of defects in a material per volume, which is generally constant at all volumes. However, when dealing with extremely small amounts of a material, there may be no defects at all, so your material may be much more conductive. In this case, you could make an argument that nearly all properties are extrinsic. But that’s not very useful, so we keep our common-sense meaning of “intrinsic properties.”
Which properties are used in materials science?
Basically, all of them. Here is a thorough (but not comprehensive list). I’ll be building additional web pages to further explain each group of properties, so if you don’t see a link below, you’ll have to check elsewhere for a more detailed explanation.
Mechanical Properties
Mechanical properties are all properties that are related to a material’s interaction with stress (force per area).
Examples of mechanical properties: strength, hardness, ductility, elasticity, toughness, creep-resistance, fatigue-resistance.
Electronic Properties
Electronic properties are all properties related to the material’s interaction with electrons. (Thus, some people might consider magnetic properties to be a subset of electronic properties). Some people might distinguish electrical properties as related to conductors, and electronic properties as related to semiconductors, but I will group them together.
Examples of electronic properties: electric conductivity/resistivity, dielectric constant, band gap, piezoelectricity, ferroelectricity, pyroelectricity, thermoelectricity, seebeck coefficient, mean free path.
Magnetic Properties
Magnetic properties are all properties related to electron spin arrangements.
Examples of magnetic properties: ferromagnetism, diamagnetism, paramagnetism, ferrimagnetism, antiferromagnetism, susceptibility, permeability, Curie temperature, Néel temperature.
Thermal Properties
Thermal properties are all properties related to atomic vibrations in the material (remember that temperature is a measure of atomic kinetic energy, i.e.vibrations).
Examples of thermal properties: thermal conductivity, coefficient of thermal expansion, Curie temperature, Néel temperature, melting point, photoelectric effect, specific heat. Notice that Curie and Néel temperature could be classified as either temperature or magnetic properties
Optical Properties
Optical properties are all properties related to light or the electromagnetic spectrum.
Examples of optical properties: speed of light in material, reflectivity, transmissivity, absorptivity, opacity/transparency, diffractivity.
Chemical Properties
Chemical properties are all properties relate to the atoms’ chemical interaction with other atoms.
Examples of chemical properties: Chemical reactivity, oxidation resistance, corrosion resistance, bond strength, adhesion, cohesion, etchability, biocompatibility, bioactivity, biodegradability, diffusivity.
Processing Properties
Processing properties are a vague set of properties related to processing techniques. These are often mechanical or thermal.
Examples of processing properties: weldability, castability, extrudability, rollability, 3D printability, machinability, recyclability.
Other Properties
I’ll just group these together, rather than making full subsets for them.
Examples: speed of sound in material, viscosity, coefficient of friction, phase changes, cost.
Properties are anything measurable about a material.
Properties are important because the material’s ultimate performance is dictated by its combination of properties.
Performance
Performance is how well a material functions in its intended role.
Performance is directly related to the material’s combination of properties. The material with the best performance is chosen for the application.
For example: which material is best to use for power lines? If you are simply looking for the best conductor, silver is the answer.
Of course, nobody would use silver for a power line because silver is way too expensive. The great conductivity is outweighed by the poor cost, so silver has an overall poor performance.
The next material that comes to mind might be copper. Copper has the 2nd highest conductivity, and it’s much cheaper, so you’re probably used to seeing copper as electrical wire in many situations. Copper has been traditionally used in power lines.
However, copper is also relatively dense (and becoming expensive). Since power lines need to be strung in the air, density and strength are quite important.
The newest power lines are typically made with a combination of steel and aluminum. Aluminum is a lightweight conductor, and steel can help carry aluminum’s weight. Both steel and aluminum are extremely cheap.
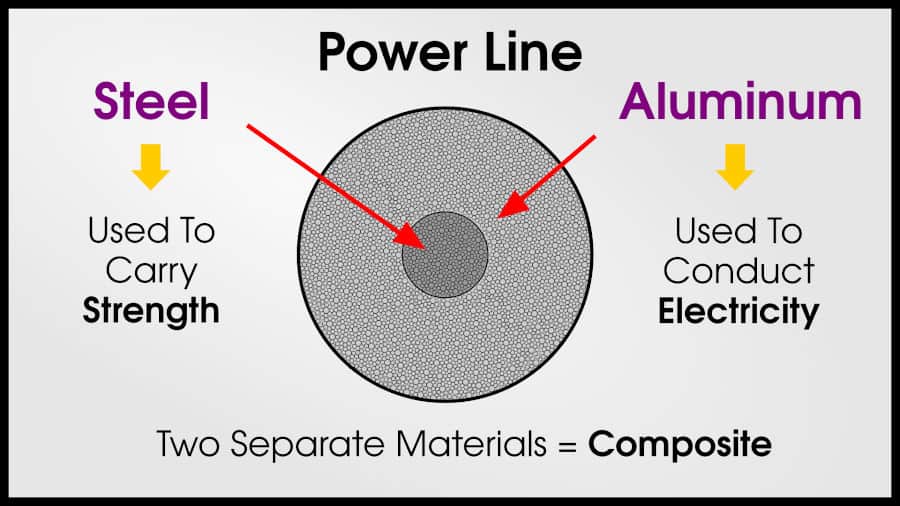
Since a composite of aluminum and steel have the best combination of strength, density, and conductivity, they have the optimal performance for power lines.
The field of materials science that most considers material performance is materials selection. Scientists and engineers working on materials selection need to consider the tradeoff between different material properties (usually weight, cost, and some additional criteria).
Performance is how well a material fulfils a certain application.
Performance is important because it’s where the money is at. Company owners and grant awards primary care about how a material will perform. If you can argue that your material will make money or improve society, you will probably get funded.
Characterization
Characterization is the act of measuring your material. That means that it’s inherently tied to properties, so I’m not sure if it really belongs in the center of the materials tetrahedron.
However, I suppose you could characterize “properties” of your processing (rather than properties of your material). You can also characterize the structure by simply observing it without necessarily measuring the properties, and you could characterize the performance of a finished product.

Characterization is the main tool which allows different points on the tetrahedron to interact.
There is destructive characterization and non-destructive characterization.
Obviously, non-destructive characterization doesn’t harm your sample and is cool because you can repeat experiments with the exact same sample. It’s also easier to convince people to let you characterize historical artifacts if you’re using non-destructive characterization.
Destructive characterization usually provides more information than non-destructive characterization, but it’s often used because it’s faster or cheaper if the material is inexpensive.
It’s also worth noting that even in non-destructive testing, samples are usually cut because they have to be relatively small.
Specialized characterization techniques have been developed to measure each set of properties listed in the properties section, but rather than go through all the different scientific equipment you’ll ever encounter, let me just list a few of the most-used ones.
Optical Microscope
OM (Optical Microscope) is one of the most basic–and oldest–scientific tools. You’ve probably used one in a high school laboratory. Modern materials science was made possible by advancing past visible light, but optical microscopes are cheap and can tell us basic information quickly.

Scanning Electron Microscope
SEM (Scanning Electron Microscope) is a microscope that uses electrons, instead of photons, to “see.” Since the wavelength of electrons is much smaller than the wavelength of visible light, SEMs allow us to observe much smaller details than OMs. Since SEMs can provide a lot of information, but are cheaper than TEMs, many materials scientists find that the SEM is their most-used tool.
Transmission Electron Microscope
TEM (Transmission Electron Microscope) is another kind of electron microscope. TEMs pass electrons directly through the sample, which results in even higher resolutions than SEMs. In the TEM, it’s possible to see individual columns of atoms. However, preparing samples for TEM is very tedious, so most people try to avoid using the machine if SEM is good enough.

X-Ray Diffraction
XRD (X-Ray Diffraction) is a technique that can measure the spacing between atoms. The machine shoots x-rays at your material, rotates the angle, and observes the diffraction angle. Bragg’s law allows us to calculate the atomic structure and spacing.
Spectroscopy
Spectroscopy measures the interaction between material and electromagnetic radiation. Four common types of spectroscopy are Raman, FTIR (Fourier-Transform Infrared Spectroscopy), EDS or EDX (Energy Dispersive X-ray Spectroscopy) and XPS (X-ray Photoelectron Spectroscopy).
Mechanical Testing
Mechanical testing is really broad and measures all the basic mechanical properties including. I’m a bit biased because I work in mechanical properties but a few examples are hardness testing, creep testing, tensile strength testing, charpy impact testing, K1C fracture toughness testing, fatigue testing, and tribology testing (friction).
Differential Scanning Calorimetry
DSC (Differential Scanning Calorimetry) is a machine that inputs thermal energy and measures the temperature change. This can measure specific heat, but more importantly, it can identify phase changes. Since changing phases often requires energy, the material will absorb the thermal energy but won’t increase in temperature. We can use this information to figure out when phase changes happen.
Thermal Gravimetric Analysis
TGA (Thermal Gravimetric Analysis) is a technique that measures the mass of your material as the temperature changes. This is especially useful for polymers that might degrade (or burn!) at high temperatures, but it can also measure weight gain from a sample oxidizing.
Atom Probe
Atom Probe is an advanced technique that most people probably won’t use, but it’s important to my research so I’m going to talk about it. The atom probe shoots a laser at your sample to blast atoms away, then it captures those atoms and digitally recreates the sample. In theory, it can tell us the position of every atom in the sample!
Characterization is measuring or observing the material.
Characterization is important because it’s how we learn information about the material.
How do the “Legs” of the Materials Tetrahedron Interact?
Materials science is defined by the space between the four corners of the materials science tetrahedron. Some scientists or engineers work more closely with one corner than the others. Many times, materials scientists are especially focused on the relationship between two points on the tetrahedron.
This is a broad generalization, but I think it’s reasonable to assign a specific job within materials science to each leg of the tetrahedron.
Structure-Properties: Materials Research
A lot of scientists working in the lab to discover more about materials are trying to figure out the relationship between structure and properties. Usually, pure research is not focused on the outcome of the properties, and they usually don’t care how the structure came to be. Research just wants to know how structure affects properties.
Processing-Structure: Materials Development
It’s called R&D (Research and Development) for a reason. These fields are usually combined. While research wants to know the reason properties exist, development wants to take that research and create better properties (via structures). Usually, research and development work together: research figures out which structures produce good properties, and development figures out how to optimize processing to create these optimal structures.
Properties-Performance: Materials Selection
Materials engineers perform “materials selection” when they tell a company which material a product should be made of. These engineers need to have a wide knowledge (or software and a database) of properties of many materials. They need to decide what combination of properties will result in the best performance.
Processing-Performance: Materials Design
Materials design hits all four points of the tetrahedron, but it focuses on the beginning and ending steps. Materials design means designing a process for a material such that the end result has the best performance. To do that requires also passing by structure and properties, of course. Materials design is a very engineering, application-minded approach to materials science.
Processing-Properties: Quality Control
Quality control is the sub-field of materials science that most operates in the processing-properties leg. Engineers performing quality control need to ensure that processes are creating the correct properties.
Structure-Performance: Failure Analysis
Engineers performing failure analysis will look at what broke and figure out why it happened. They look at some failure in the material performance, and figure out what structure caused the failure.
Remember, this list is just a helpful tool to figure out what kind of scientist or engineer has the closest relationship to a particular leg of the tetrahedron. This is not a list of all jobs within materials scientist, and it might be more fair to say that some jobs straddle a plane between three different points on the tetrahedron.
Final Thoughts
The important thing is this: every materials scientist or engineer operates within all four points of the tetrahedron. If you understand what the materials science tetrahedron is about, you will understand what materials science is about.