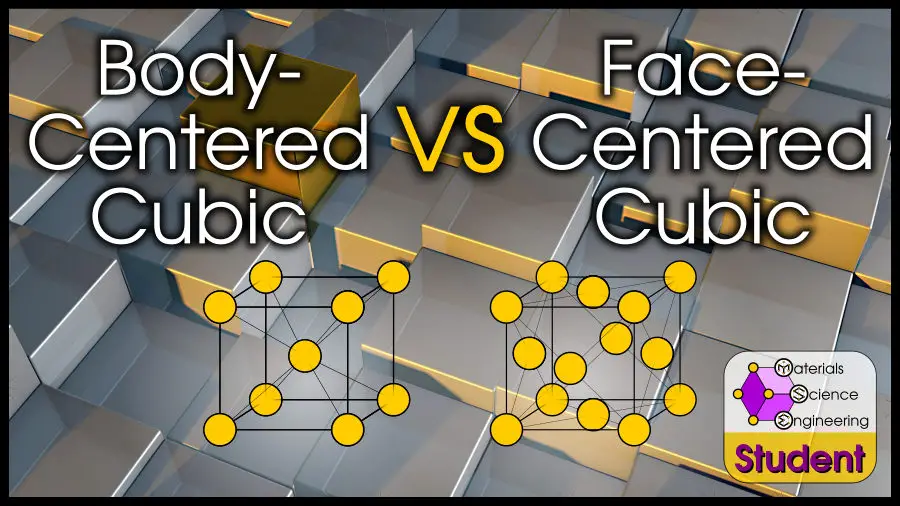
Face-centered cubic (FCC) and body-centered cubic (BCC) are two of the most iconic crystal structures. Nearly all elements have FCC, BCC, or HCP structures. You might think that–because FCC and BCC are cubic–they are much more similar than HCP. This is not the case! FCC and BCC crystals have different packing, slip systems, ductility, and more! If you’re interested, we also compared FCC and HCP crystal structures here.
The most direct difference between FCC and BCC crystals is in the atomic arrangements. The face-centered cubic structure has an atom at all 8 corner positions, and at the center of all 6 faces. The body-centered cubic structure has an atom at all 8 corner positions, and another one at the center of the cube.
FCC and BCC also have many different measurements within the unit cell, as shown in the table below.
| Crystal Structure | FCC | BCC |
| Unit Cell Type | Cubic | Cubic |
| Relationship Between Cube Edge Length a and the Atomic Radius R | a = 2R√2 | a = 4R/√3 |
| Close-Packed Structure | Yes | No |
| Atomic Packing Factor (APF) | 74% | 68% |
| Coordination Number | 12 | 8 |
| Number of Atoms per Unit Cell | 4 | 2 |
| Number of Octahedral Interstitial Sites | 4 | 6 |
| Number of Tetrahedral Interstitial Sites | 8 | 12 |
Additionally, FCC metals tend to be more dense, more stable at high temperatures, and more ductile than BCC metals. This comes from their packing arrangement and slip systems.
Outline
What Is the Packing Difference Between FCC and BCC?
This arrangement of atoms leads to another difference between FCC and BCC: atomic packing fraction. You can check this article for details about that calculation. But the result is that FCC is a more dense way of packing atoms together. (We call this APF, and you can read more about APF here). For some space, in FCC materials, about 74% of the space is occupied by atoms, leaving 26% of the space empty. In BCC materials, 68% of the space is occupied by atoms, so 32% of the space is empty.
FCC actually has the most efficient atomic arrangement possible (tied with HCP).
This is why we call FCC a “close-packed” structure. If you read a very old textbook, you may even see something called a “cubic close-packed” (CCP), which is another name for FCC.
Because FCC atoms are arranged more closely together than BCC atoms, FCC metals will tend to be more dense and more stable. This is a very broad rule, however! Tungsten, one of the densest metals, is BCC.
However, you can do one classic experiment to see the density change between BCC and FCC. If you take an iron wire–which is BCC at room temperature–and heat it up, it will transform into FCC (remember how I said that FCC is often more stable?). When it transforms, it will increase in density, so the wire will actually shrink!
Slip Systems in FCC and BCC
FCC metals tend to be more ductile than BCC metals because of their different slip systems. If you don’t understand Miller Indices, this next section will be hard to follow.
FCC crystals are close-packed along the {111} planes, and the <110> direction within that plane. Since atoms are closely packed along these directions, these are the slip planes and slip directions. Overall, we call the slip system {111}<110>. Atoms are much more likely to slip in these directions because they are closer-packed. However, the {100} system also has atoms packed close enough to be considered a valid slip system.
There are 12 total slip systems, 5 independent. If there are at least 5 independent slip systems, the metal is ductile!
BCC crystals have 48 slip systems but still only 5 are independent. Additionally, none of the BCC planes are as close-packed as the FCC planes, which generally means that BCC metals don’t slip as easily (stronger, but less ductile).
The 48 BCC slip systems are {110}<111>, {112}<111>, and {123}<111> in order of ease of activation. I’d recommend reading “Kelly & Knowles, Crystallography and Crystal Defects” if you want to prove this, and if you want to prove that only 5 of these are independent.
Further complicating BCC ductility: these slip systems may not always be active. Unlike FCC slip systems, where planes are truly close-packed, BCC slip planes don’t have atoms touching. They need thermal energy for atoms to overcome this extra distance and slip.
Depending on the element, the thermal energy to activate the slip systems will be different. At room temperature, BCC iron has all 5 slip systems activated, which is why it is ductile. If you brought iron below room temperature, however, there would not be enough thermal energy to activate all the slip systems.
At this point, iron would lose most of its ductility. We call this the ductile-to-brittle transition temperature (DBTT). All BCC metals have a ductile-to-brittle transition temperature when there is not enough thermal energy to activate 5 independent slip systems.
Because FCC has truly close-packed planes, FCC metals do not have a DBTT.
If you need materials for cryogenic applications at very low temperatures, FCC metals will usually be better than BCC metals.
Interstitial Sites in FCC and BCC
FCC and BCC crystal structure also have different arrangements of interstitial sites. Interstitial sites are the places in the unit cell between atoms. You already know that unit cells of metals are not fully packed (74% for FCC and 68% for BCC), which means they have some empty space.
Smaller atoms can fit in these spaces. Because of the different interstitial sites, different atoms can form different interstitial alloys depending on the crystal structure.
As a rule of thumb, atoms that fit in interstitial sites should be less than 15% of the size of atoms in regular sites. In fact, the exact radius ratio also determines which kind of interstitial site can be occupied.
The two main types of interstitial sites are octahedral and tetrahedral.
These sites are named because of their nearest neighbors. Octahedral sites have 6 nearest neighbors, and tetrahedral sites have 4 nearest neighbors.
Octahedral sites are much bigger in FCC than in BCC. However, tetrahedral sites are larger in BCC, and BCC has more tetrahedral and octahedral sites.
| Crystal Structure | FCC | BCC |
| Number and Size of Octahedral Voids | 4 voids, r = 0.414 R | 6 voids, r = 0.155 R |
| Number and Size of Tetrahedral Voids | 8 voids, r = 0.225 R | 12 voids, r = 0.291 R |
FCC: octahedral sites bigger than tetrahedral
BCC: tetrahedral sites bigger than octahedral
Although BCC has more total room for interstitial atoms, FCC has the largest particular interstitial site (octahedral). This can have a large impact in interstitial solubility.
For example, iron is BCC at room temperature (we call this phase “ferrite”) but FCC at higher temperatures (we call this phase “austenite”). Because of carbon’s size relative to iron, it wants to fit into octahedral sites. In fact, carbon barely fits into BCC iron–its solubility is only 0.02 wt%. In contrast, 100x that amount of carbon can dissolve in FCC iron.
This fact, actually, is one of the reasons why steel (iron and carbon alloy) is so useful. It’s possible to make a very hard phase in steel which does not appear on a phase diagram. If you heat steel up in the presence of carbon (like coal or charcoal), the steel becomes FCC and more carbon will dissolve in the lattice.
When this FCC steel is cooled rapidly (quenched), the carbon does not have time to diffuse out of the lattice. Instead of BCC steel, you end up with body-centered tetragonal (BCT). The extra carbon gets trapped in the lattice and distorts the normally cubic lattice. We call this highly strained phase “martensite” and it’s the phase present in hard steels (like swords).
Octahedral Sites
There are 1 + 12/4 = 4 octahedral site positions per unit cell in the FCC crystal structure.
There are 6/2 + 12/4 = 6 octahedral site positions per unit cell in the BCC crystal structure.
Tetrahedral Sites
There are 8 octahedral site positions per unit cell in the FCC crystal structure.
There are (6×4)/2 = 12 octahedral site positions per unit cell in the BCC crystal structure.
Examples of FCC and BCC Elements
At room temperature, some common FCC metals are aluminum, nickel, and copper. Some common BCC metals are chromium, iron, and tungsten.
Here is a list of all the elements which are FCC, BCC, or HCP at room temperature.
Face-Centered Cubic (FCC) elements:
Body-Centered Cubic (BCC) elements:
Hexagonal Close-Packed (HCP) elements:
In general, alloys with these metals will have the same crystal structure as the most common element. However, there are always exceptions. (For example, cobalt and iron can both be FCC when heavily alloyed, especially with nickel).
Final Thoughts
Now you know all the differences between FCC and BCC! You also know many common materials that take each form. Now you see, even though FCC and BCC are both cubic, they have many differences because BCC is not close-packed.
References and Further Reading
If you’re reading this article as an introductory student in materials science, welcome! I hope you can find many other useful articles on this website.
If you’re reading this article because you’re taking a class on structures, you may be interested in my other crystallography articles. Here is this list, in recommended reading order:
Introduction to Bravais Lattices
What is the Difference Between “Crystal Structure” and “Bravais Lattice”
Atomic Packing Factor
How to Read Miller Indices
How to Read Hexagonal Miller-Bravais Indices
Close-Packed Crystals and Stacking Order
Interstitial Sites
Primitive Cells
How to Read Crystallography Notation
What are Point Groups
List of Point Groups
If you are interested in more details about any specific crystal structure, I have written individual articles about simple crystal structures which correspond to each of the 14 Bravais lattices:
1. Simple Cubic
2. Face-Centered Cubic
2a. Diamond Cubic
3. Body-Centered Cubic
4. Simple Hexagonal
4a. Hexagonal Close-Packed
4b. Double Hexagonal Close-Packed (La-type)
5. Rhombohedral
5a. Rhombohedral Close-Packed (Sm-type)
6. Simple Tetragonal
7. Body-Centered Tetragonal
7a. Diamond Tetragonal (White Tin)
8. Simple Orthorhombic
9. Base-Centered Orthorhombic
10. Face-Centered Orthorhombic
11. Body-Centered Orthorhombic
12. Simple Monoclinic
13. Base-Centered Monoclinic
14. Triclinic